
ORGANIC CHEMISTRY-PRINT (LL)-W/WILEY
4th Edition
ISBN: 9781119761105
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 25, Problem 73PP
Interpretation Introduction
Interpretation:
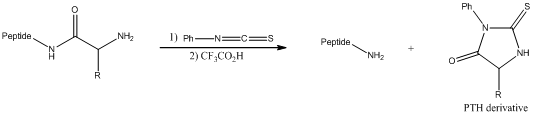
Structure of the tripeptide considered need to be drawn from the given products obtained.
Concept introduction:
Edman degradation is a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Can the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left?
?
starting
material
target
If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area.
Be sure you follow the standard ALEKS rules for submitting syntheses.
+ More...
Note for advanced students: you may assume that you are using a large excess of benzene as your starting material.
C
:0
T
Add/Remove step
G
The following equations represent the formation of compound MX. What is the AH for the
electron affinity of X (g)?
X₂ (g) → 2X (g)
M (s) → M (g)
M (g)
M (g) + e-
AH = 60 kJ/mol
AH = 22 kJ/mol
X (g) + e-X (g)
M* (g) +X (g) → MX (s)
AH = 118 kJ/mol
AH = ?
AH = -190 kJ/mol
AH = -100 kJ/mol
a)
-80 kJ
b)
-30 kJ
c)
-20 kJ
d)
20 kJ
e)
156 kJ
A covalent bond is the result of the
a)
b)
c)
d)
e)
overlap of two half-filled s orbitals
overlap of a half-filled s orbital and a half-filled p orbital
overlap of two half-filled p orbitals along their axes
parallel overlap of two half-filled parallel p orbitals
all of the above
Chapter 25 Solutions
ORGANIC CHEMISTRY-PRINT (LL)-W/WILEY
Ch. 25.2 - Prob. 1CCCh. 25.2 - Prob. 2CCCh. 25.2 - Prob. 3CCCh. 25.2 - Prob. 1LTSCh. 25.2 - Prob. 4PTSCh. 25.2 - Prob. 7CCCh. 25.2 - Prob. 8CCCh. 25.2 - Prob. 9CCCh. 25.2 - Prob. 10CCCh. 25.2 - Prob. 11CC
Ch. 25.3 - Prob. 12CCCh. 25.3 - Prob. 13CCCh. 25.3 - Prob. 2LTSCh. 25.3 - Prob. 14PTSCh. 25.3 - Prob. 17CCCh. 25.3 - Prob. 18CCCh. 25.3 - Prob. 19CCCh. 25.3 - Prob. 20CCCh. 25.4 - Prob. 3LTSCh. 25.4 - Prob. 21PTSCh. 25.4 - Prob. 25CCCh. 25.4 - Prob. 26CCCh. 25.4 - Prob. 27CCCh. 25.4 - Prob. 28CCCh. 25.4 - Prob. 29CCCh. 25.5 - Prob. 30CCCh. 25.5 - Prob. 4LTSCh. 25.5 - Prob. 31PTSCh. 25.6 - Prob. 5LTSCh. 25.6 - Prob. 34PTSCh. 25 - Prob. 40PPCh. 25 - Prob. 41PPCh. 25 - Prob. 42PPCh. 25 - Prob. 43PPCh. 25 - Prob. 44PPCh. 25 - Prob. 45PPCh. 25 - Prob. 46PPCh. 25 - Prob. 47PPCh. 25 - Prob. 48PPCh. 25 - Prob. 49PPCh. 25 - Prob. 50PPCh. 25 - Prob. 51PPCh. 25 - Prob. 52PPCh. 25 - Prob. 53PPCh. 25 - Prob. 54PPCh. 25 - Prob. 55PPCh. 25 - Prob. 56PPCh. 25 - Prob. 57PPCh. 25 - Prob. 58PPCh. 25 - Prob. 59PPCh. 25 - Prob. 60PPCh. 25 - Prob. 61PPCh. 25 - Prob. 62PPCh. 25 - Prob. 63PPCh. 25 - Prob. 64PPCh. 25 - Prob. 65PPCh. 25 - Prob. 66PPCh. 25 - Prob. 67PPCh. 25 - Prob. 68PPCh. 25 - Prob. 69PPCh. 25 - Prob. 70PPCh. 25 - Prob. 71PPCh. 25 - Prob. 72PPCh. 25 - Prob. 73PPCh. 25 - Prob. 74PPCh. 25 - Prob. 75PPCh. 25 - Prob. 76PPCh. 25 - Prob. 77PPCh. 25 - Prob. 78PPCh. 25 - Prob. 79PPCh. 25 - Prob. 80PPCh. 25 - Prob. 81PPCh. 25 - Prob. 82PPCh. 25 - Prob. 83PPCh. 25 - Prob. 89IPCh. 25 - Prob. 90IPCh. 25 - Prob. 91IPCh. 25 - Prob. 92IP
Knowledge Booster
Similar questions
- Can the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forwardWhich one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forwardAll of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forward
- A student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forwardPredict the major products of this organic reaction:arrow_forwardCalculate the density of 21.12 g of an object that displaces 0.0250 L of water.arrow_forward
- Draw the expected reactant R28. Cu(II) CO₂Mearrow_forwardPpplllleeeaaasssseeee helllppp wiithhh thisss Organic chemistryyyyyy I talked like this because AI is very annoyingarrow_forwardName the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward
- 1b. Br LOHarrow_forwardI would like my graphs checked please. Do they look right? Do I have iodine and persulfate on the right axis ?arrow_forwardReaction Fill-ins Part 2! Predict the product(s) OR starting material of the following reactions. Remember, Hydride shifts are possible if/when a more stable carbocation can exist (depending on reaction mechanism)! Put your answers in the indicated boxes d. d. ง HCIarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY