
EBK ORGANIC CHEMISTRY
7th Edition
ISBN: 9780133556186
Author: Bruice
Publisher: VST
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 24.5, Problem 15P
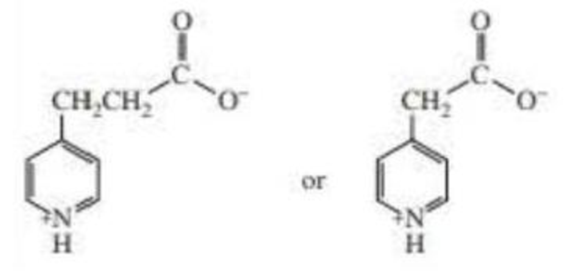
Which compound is more easily decarboxylated?
Expert Solution & Answer
Learn your wayIncludes step-by-step video

schedule03:18
Students have asked these similar questions
34. Figure 3 shows Van Deemter plots for a solute molecule using different column inner diameters (i.d.).
A) Predict whether decreasing the column inner diameters increase or decrease bandwidth.
B) Predict which van Deemter equation coefficient (A, B, or C) has the greatest effect on increasing or
decreasing bandwidth as a function of i.d. and justify your answer.
Figure 3 Van Deemter plots for hydroquinone using different column inner diameters (i.d. in μm). The data was
obtained from liquid chromatography experiments using fused-silica capillary columns packed with 1.0-μm particles.
35
20
H(um)
큰 20
15
90
0+
1500
100
75
550
01
02
594
05
μ(cm/sec)
30
15
10
elow are
experimentally determined van Deemter plots of column efficiency, H, vs. flow rate. H is a
quantitative measurement of band broadening. The left plot is for a liquid chromatography application and the
night is for gas chromatography. Compare and contrast these two plots in terms of the three band broadening
mechanisms presented in this activity. How are they similar? How do they differ? Justify your answers.?
0.4
H (mm)
0.2
0.1-
0.3-
0
0.5
H (mm)
8.0
7.0
6.0
5.0
4.0-
3.0
T
+++
1.0
1.5
0
2.0
4.0
Flow Rate, u (cm/s)
6.0
8.0
Flow Rate, u (cm/s)
Predict the products of this organic reaction:
+
H
ZH
NaBH3CN
H+
n.
?
Click and drag to start drawing a
structure.
X
Chapter 24 Solutions
EBK ORGANIC CHEMISTRY
Ch. 24.1 - Prob. 2PCh. 24.1 - Prob. 3PCh. 24.2 - How many conjugated double bonds are there in a....Ch. 24.2 - Instead of adding to the 4a position and...Ch. 24.2 - Prob. 7PCh. 24.3 - Prob. 8PCh. 24.3 - Acetolactate synthase is another TPP-requiring...Ch. 24.3 - Acetolactate synthase transfers the acyl group of...Ch. 24.5 - Prob. 12PCh. 24.5 - The enzyme that catalyzes the C C bond cleavage...
Ch. 24.5 - Propose a mechanism for the ,-elimination reaction...Ch. 24.5 - Which compound is more easily decarboxylated?Ch. 24.5 - Explain why the ability of PLP to catalyze an...Ch. 24.5 - Explain why the ability of PLP to catalyze an...Ch. 24.6 - Ethanolamine ammonia lyase, a coenzyme...Ch. 24.6 - Prob. 19PCh. 24.7 - How do the structure of tetrahydrofolate and...Ch. 24.7 - What is the source of the methyl group in...Ch. 24.8 - Thiols such as ethanethiol and propanethiol can be...Ch. 24 - How does the metal ion in carboxypeptidase A...Ch. 24 - Prob. 23PCh. 24 - Prob. 24PCh. 24 - Prob. 25PCh. 24 - S-Adenosylmethionine (SAM) is formed from the...Ch. 24 - Prob. 27PCh. 24 - What acyl groups have we seen transferred by...Ch. 24 - Propose a mechanism for the following reaction:Ch. 24 - When transaminated, the three branched-chain amino...Ch. 24 - Draw the products of the following reaction, where...Ch. 24 - When UMP is dissolved in T2O, exchange of T for H...Ch. 24 - Dehydratase is a PLP-requiring enzyme that...Ch. 24 - In addition to the reaction mentioned in Section...Ch. 24 - PLP can catalyze both ,-elimination reactions...Ch. 24 - The glycine cleavage system is a group of four...Ch. 24 - Prob. 37PCh. 24 - FADH2 reduces , -unsaturated thioesters to...
Additional Science Textbook Solutions
Find more solutions based on key concepts
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
How can the freezing of water crack boulders?
Campbell Biology in Focus (2nd Edition)
Pigeons may exhibit a checkered or plain color pattern. In a series of controlled matings, the following data w...
Concepts of Genetics (12th Edition)
[14.110] The following mechanism has been proposed for the gas-phase reaction of chloroform (CHCI3) and chlorin...
Chemistry: The Central Science (14th Edition)
15. In the Olympic shotput event, an athlete throws the shot with an initial speed of 12.0 m/s at a 40.0° angle...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
16. Explain some of the reasons why the human species has been able to expand in number and distribution to a g...
Campbell Biology: Concepts & Connections (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the missing reactant R in this organic reaction? + R H3O+ + • Draw the structure of R in the drawing area below. • Be sure to use wedge and dash bonds if it's necessary to draw one particular enantiomer. Click and drag to start drawing a structure.arrow_forwardWhat would be the best choices for the missing reagents 1 and 3 in this synthesis? 1 1. PPh3 2. n-BuLi 2 • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Click and drag to start drawing a structure.arrow_forwardThe product on the right-hand side of this reaction can be prepared from two organic reactants, under the conditions shown above and below the arrow. Draw 1 and 2 below, in any arrangement you like. 1+2 NaBH₂CN H+ N Click and drag to start drawing a structure. X $arrow_forward
- Explain what is the maximum absorbance of in which caffeine absorbs?arrow_forwardExplain reasons as to why the amount of caffeine extracted from both a singular extraction (5ml Mountain Dew) and a multiple extraction (2 x 5.0ml Mountain Dew) were severely high when compared to coca-cola?arrow_forwardProtecting Groups and Carbonyls 6) The synthesis generates allethrolone that exhibits high insect toxicity but low mammalian toxicity. They are used in pet shampoo, human lice shampoo, and industrial sprays for insects and mosquitos. Propose detailed mechanistic steps to generate the allethrolone label the different types of reagents (Grignard, acid/base protonation, acid/base deprotonation, reduction, oxidation, witting, aldol condensation, Robinson annulation, etc.) III + VI HS HS H+ CH,CH,Li III I II IV CI + P(Ph)3 V ༼ Hint: no strong base added VI S VII IX HO VIII -MgBr HgCl2,HgO HO. isomerization aqeuous solution H,SO, ༽༽༤༽༽ X MeOH Hint: enhances selectivity for reaction at the S X ☑arrow_forward
- Draw the complete mechanism for the acid-catalyzed hydration of this alkene. esc 田 Explanation Check 1 888 Q A slock Add/Remove step Q F4 F5 F6 A བྲA F7 $ % 5 @ 4 2 3 & 6 87 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce W E R T Y U S D LL G H IK DD 요 F8 F9 F10 F1 * ( 8 9 0 O P J K L Z X C V B N M H He commandarrow_forwardExplanation Check F1 H₂O H₂ Pd 1) MCPBA 2) H3O+ 1) Hg(OAc)2, H₂O 2) NaBH4 OH CI OH OH OH hydration halohydrin formation addition halogenation hydrogenation inhalation hydrogenation hydration ☐ halohydrin formation addition halogenation formation chelation hydrogenation halohydrin formation substitution hydration halogenation addition Ohalohydrin formation subtraction halogenation addition hydrogenation hydration F2 80 F3 σ F4 F5 F6 1 ! 2 # 3 $ 4 % 05 Q W & Å © 2025 McGraw Hill LLC. All Rights Reserved. F7 F8 ( 6 7 8 9 LU E R T Y U A F9arrow_forwardShow the mechanism steps to obtain the lowerenergy intermediate: *see imagearrow_forward
- Soap is made by the previous reaction *see image. The main difference between one soap and another soap isthe length (number of carbons) of the carboxylic acid. However, if a soap irritates your skin, they mostlikely used too much lye.Detergents have the same chemical structure as soaps except for the functional group. Detergentshave sulfate (R-SO4H) and phosphate (R-PO4H2) functional groups. Draw the above carboxylic acidcarbon chain but as the two variants of detergents. *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License