
Concept explainers
(a)
Interpretation: The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is to be shown. The reagents that are needed to convert the obtained product to the given compound are to be predicted.
Concept introduction: Aldol reaction is the condensation reaction of the
Answer to Problem 24.9P
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is
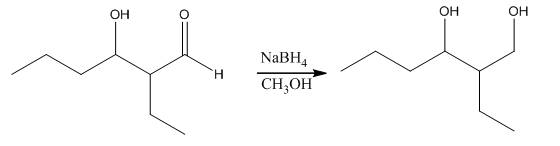
Explanation of Solution
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is shown as,
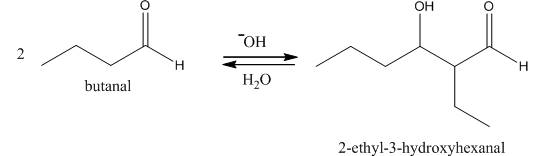
Figure 1
In this reaction, first of all, one equivalent of butanal is treated with the strong base that results in the formation of a resonance-stabilized enolate ion. This enolate ion reacts with the second equivalent of butanal followed by the hydrolysis that leads to the formation of the desired product,
The reagents that are needed to convert

Figure 2
Thus, the reagent that is needed to convert
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is
(b)
Interpretation: The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is to be shown. The reagents that are needed to convert the obtained product to the given compound are to be predicted.
Concept introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 24.9P
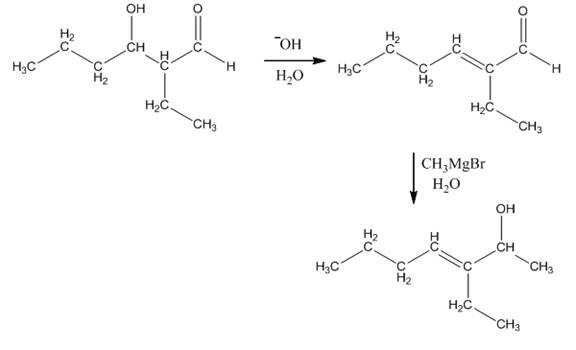
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is
Explanation of Solution
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is shown in Figure 1.
In this reaction, first of all, one equivalent of butanal is treated with the strong base that results in the formation of a resonance-stabilized enolate ion. This enolate ion reacts with the second equivalent of butanal followed by the hydrolysis that leads to the formation of the desired product,
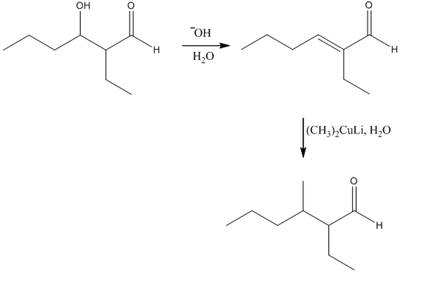
The reagents that are needed to convert
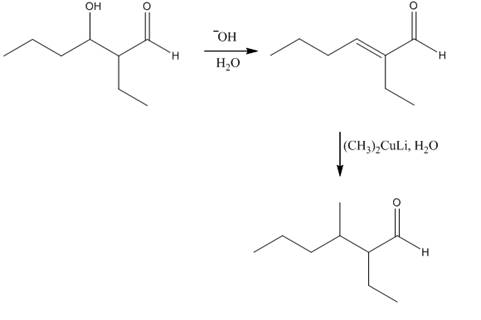
Figure 3
In this reaction,
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is
(c)
Interpretation: The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is to be shown. The reagents that are needed to convert the obtained product to the given compound are to be predicted.
Concept introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 24.9P
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is
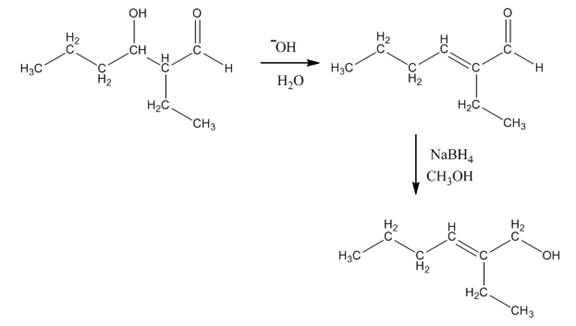
Explanation of Solution
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is shown in Figure 1.
In this reaction, first of all, one equivalent of butanal is treated with the strong base that results in the formation of a resonance-stabilized enolate ion. This enolate ion reacts with the second equivalent of butanal followed by the hydrolysis that leads to the formation of the desired product,
The reagents that are needed to convert
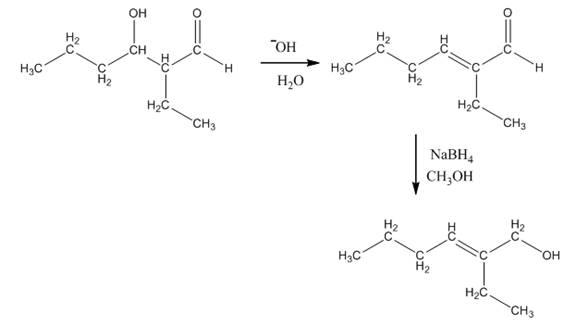
Figure 4
In this reaction,
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is
(d)
Interpretation: The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is to be shown. The reagents that are needed to convert the obtained product to the given compound are to be predicted.
Concept introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 24.9P
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is
Explanation of Solution
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is shown in Figure 1.
In this reaction, first of all, one equivalent of butanal is treated with the strong base that results in the formation of a resonance-stabilized enolate ion. This enolate ion reacts with the second equivalent of butanal followed by the hydrolysis that leads to the formation of the desired product,
The reagents that are needed to convert

Figure 5
In this reaction,
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is
Want to see more full solutions like this?
Chapter 24 Solutions
Organic Chemistry-Package(Custom)
- Indicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forward
- Synthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forward
- Indicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


