
Concept explainers
(a)
Interpretation: The product that is formed by the aldol reaction of the given starting material(s) by using
Concept introduction: Aldol reaction is the condensation reaction of the
Answer to Problem 24.28P
The product that is formed by the aldol reaction of the given starting material(s) by using
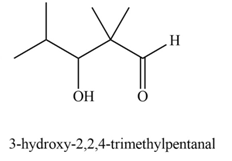
Explanation of Solution
The product that is formed by the aldol reaction of the given starting material(s) by using
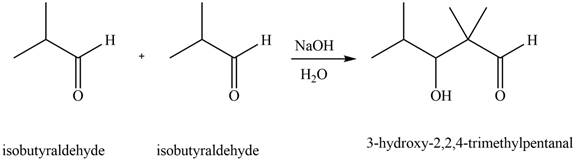
Figure 1
In this reaction, one molecule of isobutyraldehyde is treated with the strong base that leads to the formation of a resonance-stabilized enolate ion. Then, this enolate ion reacts with the second molecule of isobutyraldehyde followed by the hydrolysis to form
The product that is formed by the aldol reaction of the given starting material(s) by using
(b)
Interpretation: The product that is formed by the aldol reaction of the given starting material(s) by using
Concept introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 24.28P
The product that is formed by the aldol reaction of the given starting material(s) by using
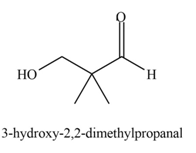
Explanation of Solution
The product that is formed by the aldol reaction of the given starting material(s) by using
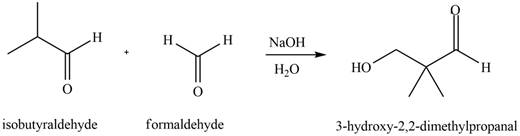
Figure 2
In this reaction, the compound, isobutyraldehyde is treated with the strong base that leads to the formation of a resonance-stabilized enolate ion. Then, this enolate ion reacts with formaldehyde followed by the hydrolysis to form
The product that is formed by the aldol reaction of the given starting material(s) by using
(c)
Interpretation: The product that is formed by the aldol reaction of the given starting material(s) by using
Concept introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 24.28P
The product that is formed by the aldol reaction of the given starting material(s) by using
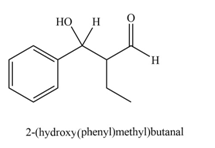
Explanation of Solution
The product that is formed by the aldol reaction of the given starting material(s) by using
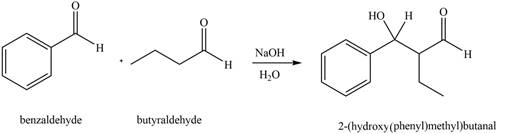
Figure 3
In this reaction, the compound, butyraldehyde is treated with the strong base that leads to the formation of a resonance-stabilized enolate ion. Then, this enolate ion reacts with benzaldehyde followed by the hydrolysis to form
The product that is formed by the aldol reaction of the given starting material(s) by using
(d)
Interpretation: The product that is formed by the aldol reaction of the given starting material(s) by using
Concept introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 24.28P
The product that is formed by the aldol reaction of the given starting material(s) by using
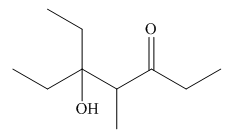
Explanation of Solution
The product that is formed by the aldol reaction of the given starting material(s) by using
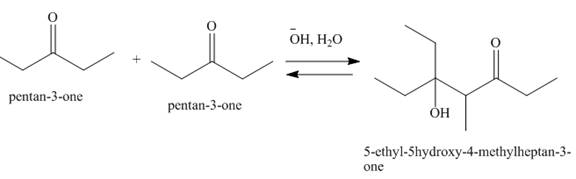
Figure 4
In this reaction, one molecule of pentan-
The product that is formed by the aldol reaction of the given starting material(s) by using
Want to see more full solutions like this?
Chapter 24 Solutions
Organic Chemistry-Package(Custom)
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





