
(a)
Interpretation:
The other products obtained from the highly stereo and regioselective reaction of O-alkylation during the formation of Gilvarin M. has to be interpreted.
Concept introduction:
Regioselectivity:
Regioselectivity is the preference of one direction of
Stereoselectivity:
Stereoselectivity is the property of a
(a)
Explanation of Solution
The reaction is given as,
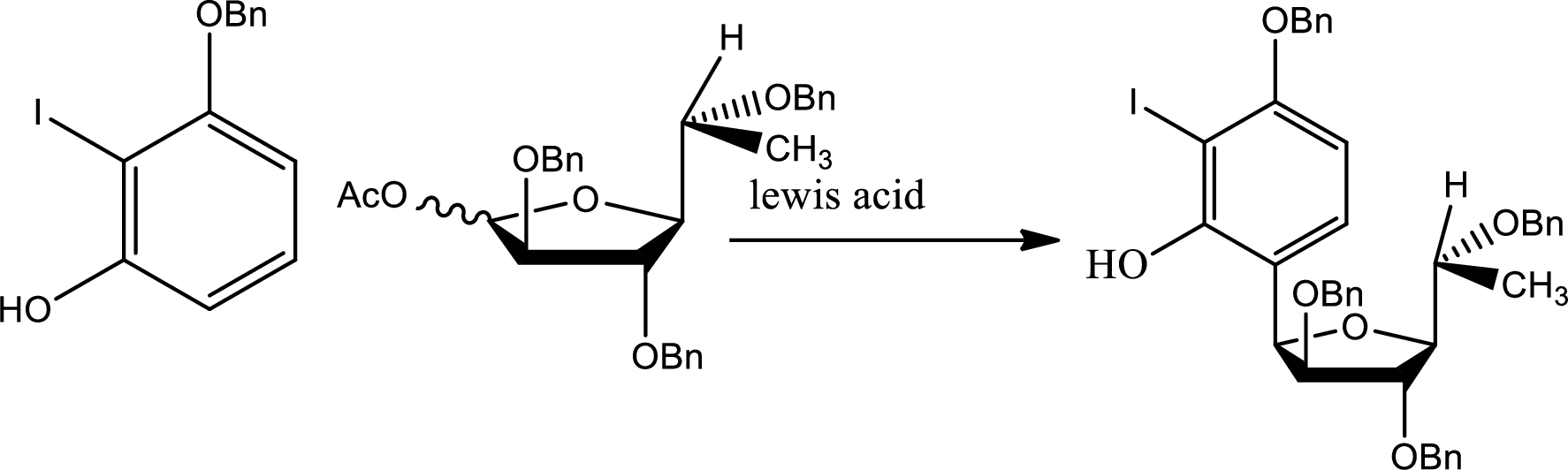
The other possibility is,
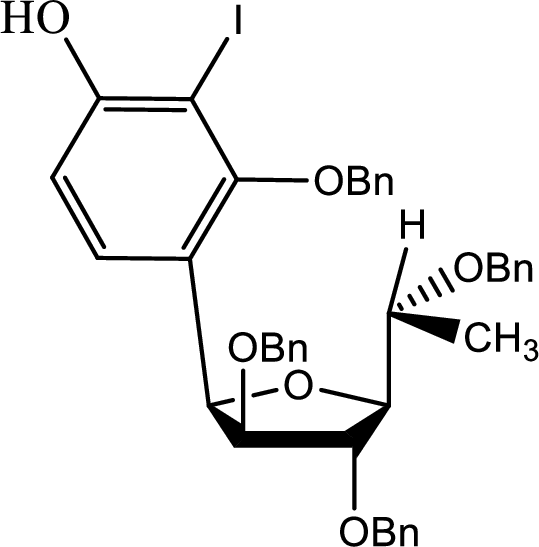
(b)
Interpretation:
The structure of the compound C has to be given.
Concept introduction:
Acid-base reaction:
The species that donates proton or accepts lone pair of electrons are called acids and those who accepts proton or donates lone pair of electrons are called base.
The
(b)
Explanation of Solution
The pathway of formation of C is,
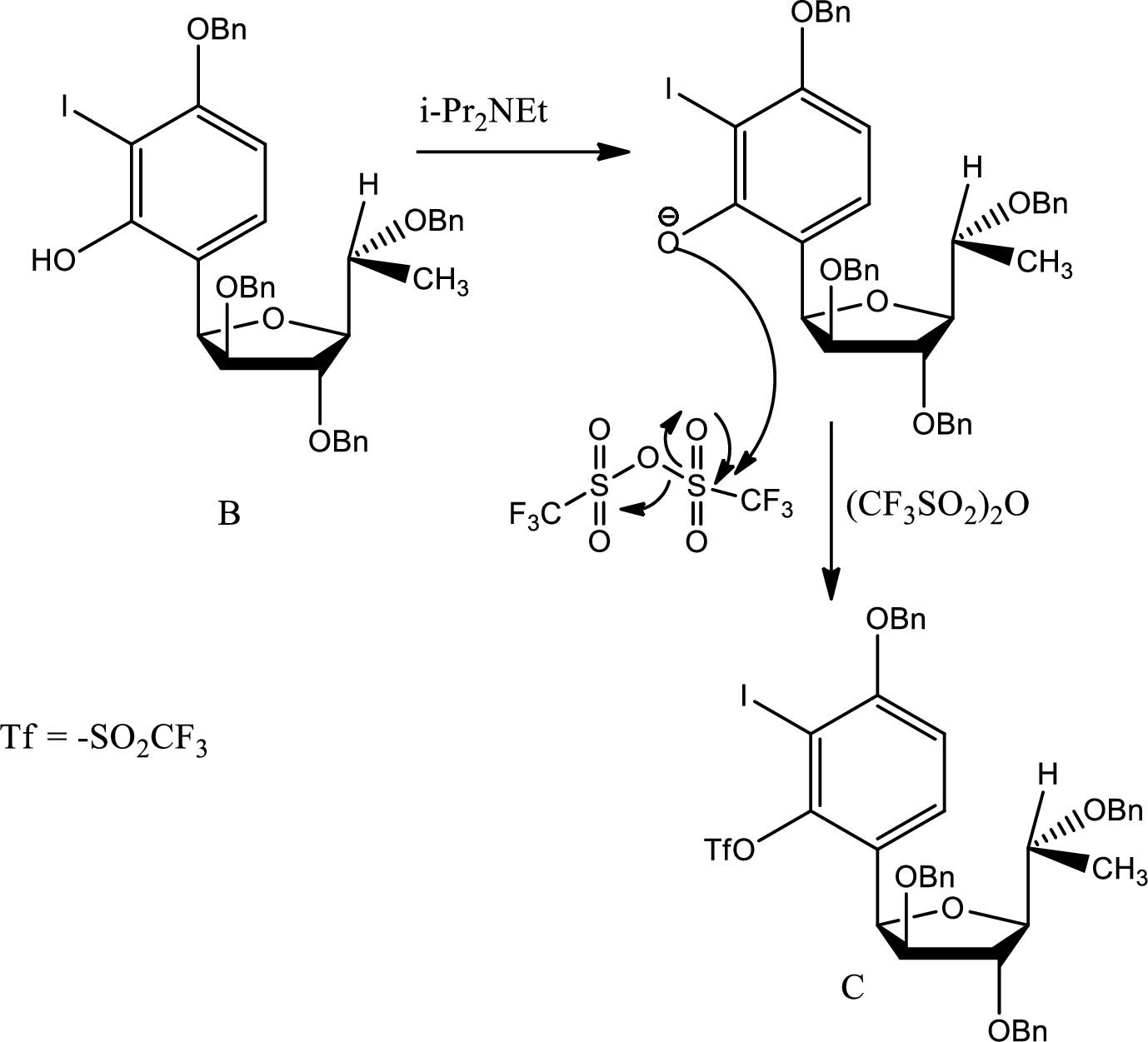
Here the
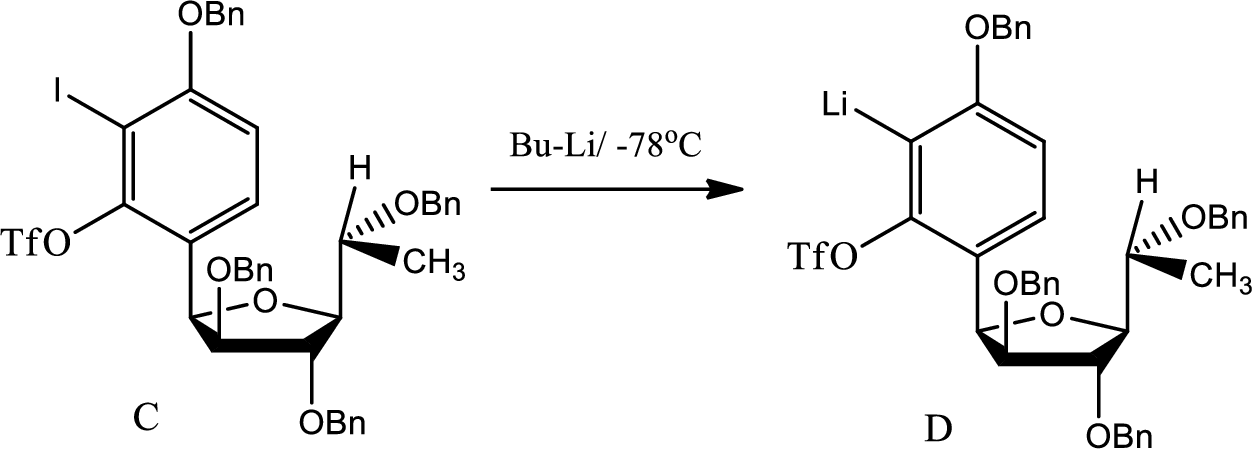
(c)
Interpretation:
The structure of compound D has to be given.
Concept introduction:
Lithium halogen exchange:
Organolithium reagents are characterised by the presence of
The reaction of lithium metal at low temperature with an
The same reaction happens with the haloarenes also,
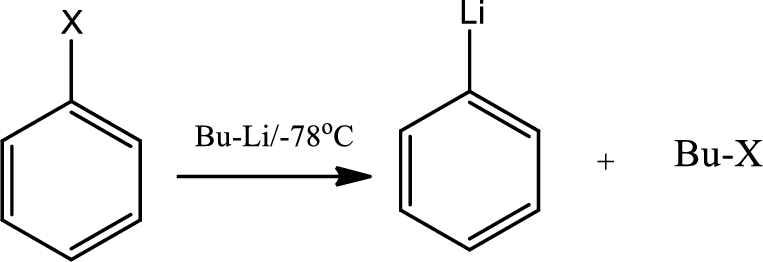
(c)
Explanation of Solution
The reaction for the formation of D is as follows,
(d)
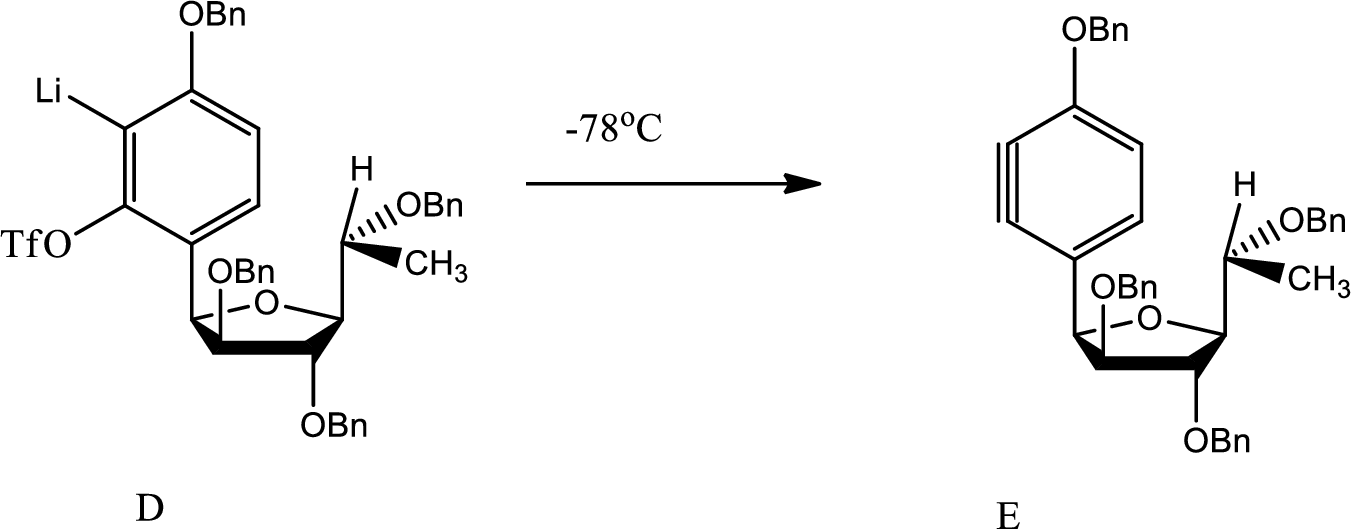
Interpretation:
Structure of E has to be given along with the mechanism of formation of E from D.
Concept introduction:
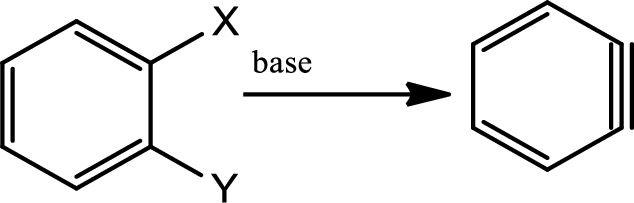
Benzyne formation:
Benzynes are highly reactive intermediate species that are made from
(d)
Explanation of Solution
Here benzyne formation occurs due to removal of
(e)
Interpretation:
A mechanism for formation of F from E has to be given.
Concept introduction:
Diels Alder reaction:
The Diels-Alder reaction is a chemical reaction between a conjugated diene and a substituted
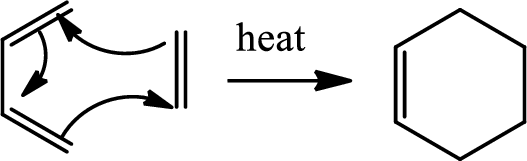
(e)
Explanation of Solution
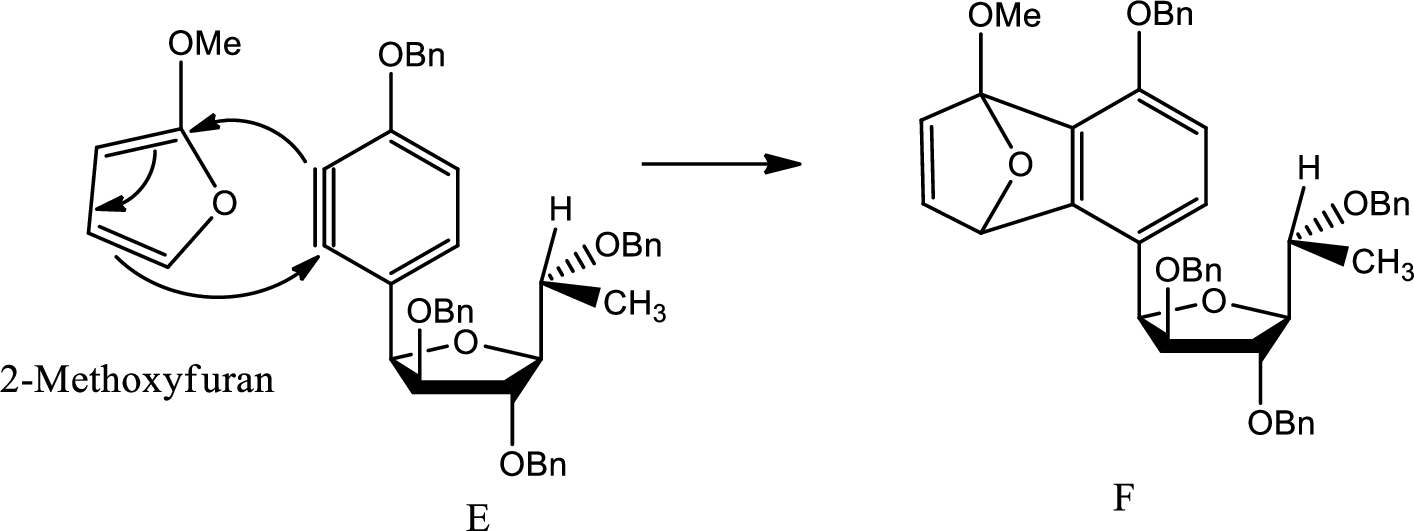
Here Diels Alder reaction occurs between furan and benzyne. A new ring is formed.
(f)
Interpretation:
Mechanism from F to G has to be given.
Concept introduction:
Hydrolysis:
Hydrolysis is any chemical reaction in which a water molecule ruptures one or more chemical bonds. This is mainly used for substitution, elimination and fragmentation reactions in which water is the nucleophile. Hydrolysis is reverse of condensation reaction because in this process water is added to beak down.
(f)
Explanation of Solution
Here aqueous work up leads to breaking of the bridging oxygen bond by protonating the oxygen. Thus the angle strain increases as positive charge on electronegative oxygen is tough. Thus the bond breaks and the charge formed is stabilised by electron donating effect of
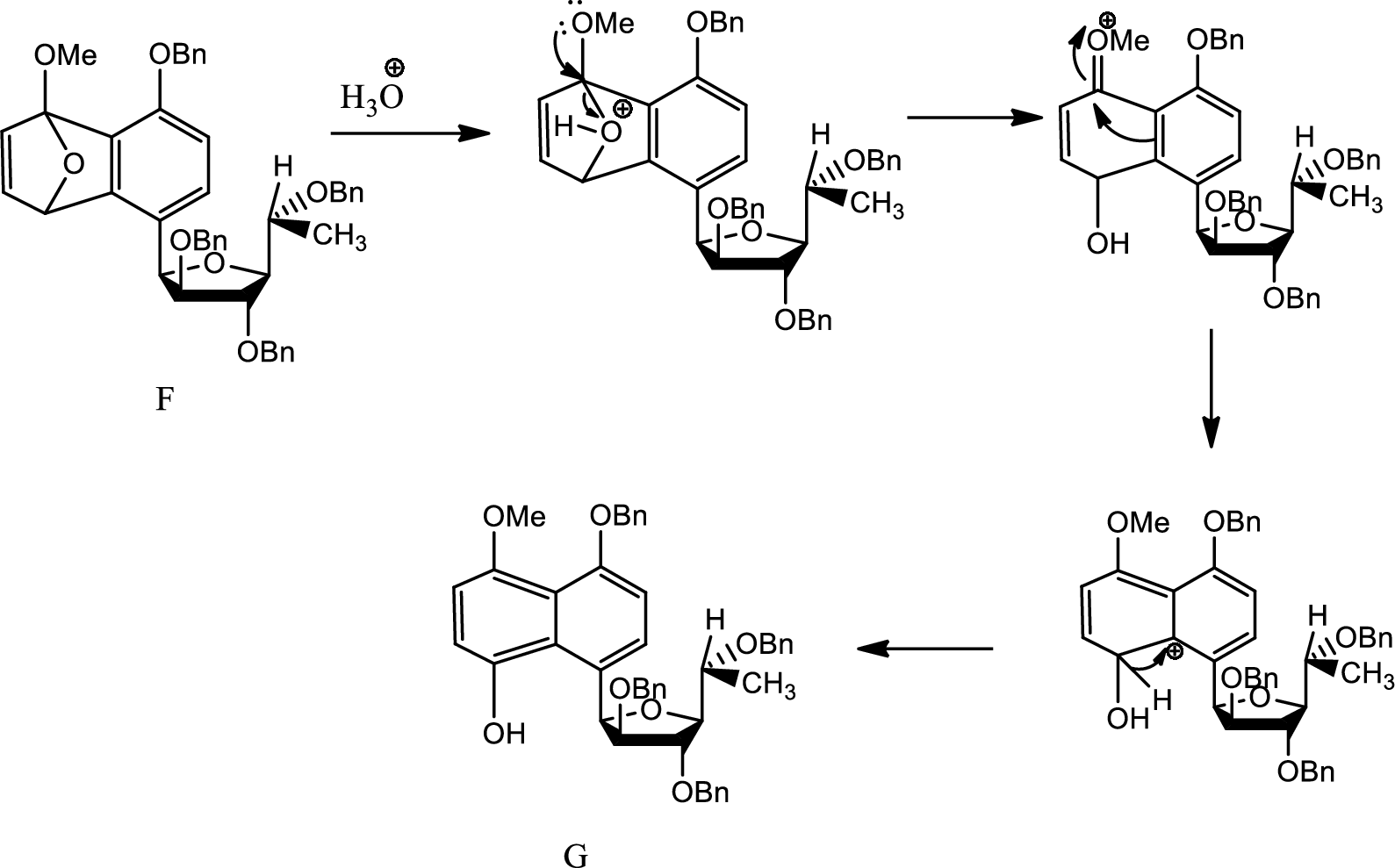
Thus G is formed from F.
(g)
Interpretation:
The reagents and condition from G to H has to be given.
Concept introduction:
Acid-base reaction:
The species that donates proton or accepts lone pair of electrons are called acids and those who accepts proton or donates lone pair of electrons are called base.
The
(g)
Explanation of Solution
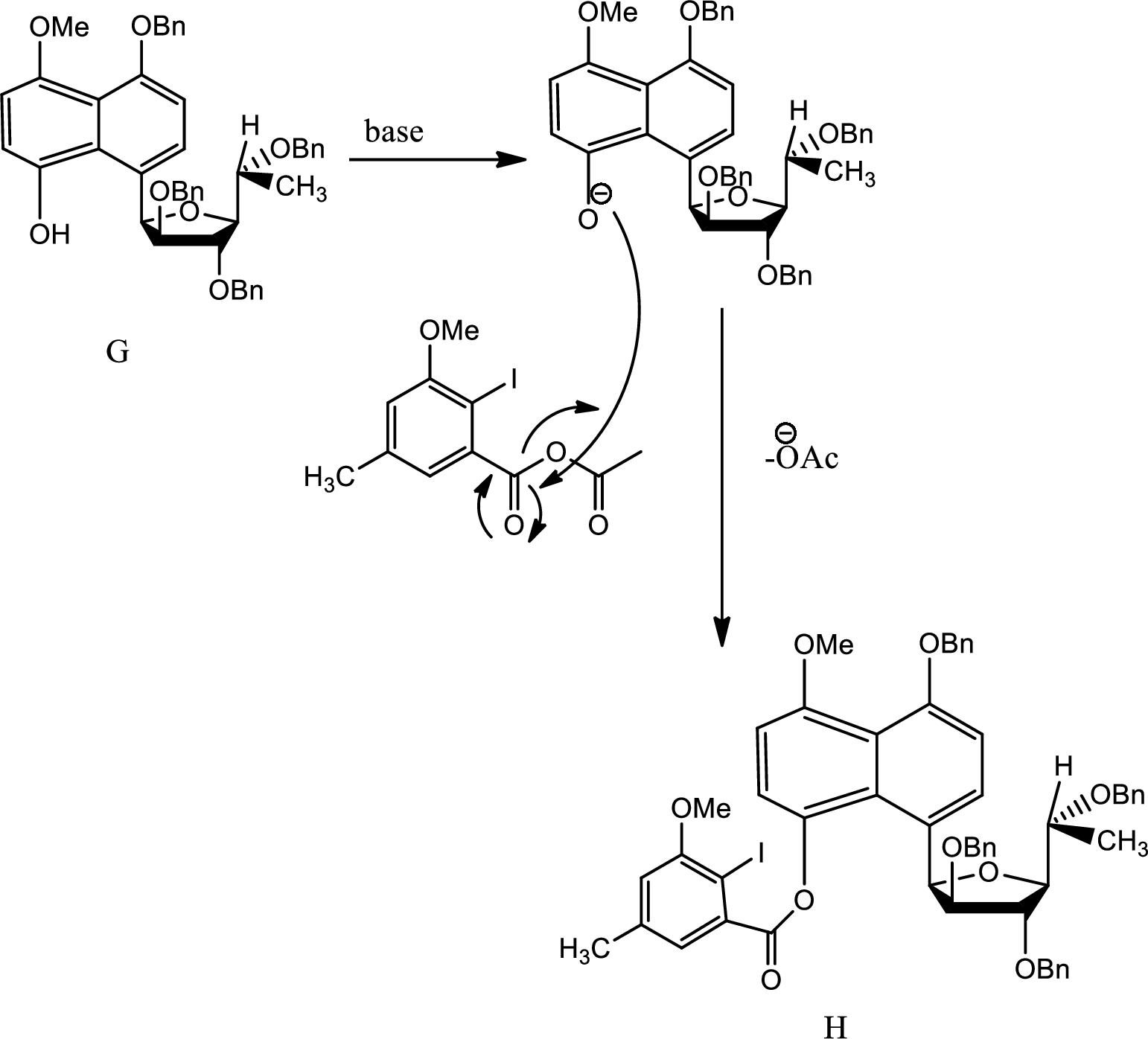
Here on giving base the acidic hydrogen from phenol is removed and the new carbanion formed undergoes
Thus H is formed from G.
(h)
Interpretation:
Reagents and condition for conversion of H to I has to be given.
Concept introduction:
Cross coupling:
A cross coupling reaction is defined as a reaction that creates a
In the case of palladium catalysed cross-coupling reactions the other metal or metalloids are commonly
Suzuki coupling:
The Suzuki coupling uses a boron compound and an alkenyl, aryl or alkyl halide or triflate as the carbon sources with a palladium salt as a catalyst. The reaction is mainly used to form biaryls. The mechanism of the reaction starts with an oxidative addition followed by transmetallation in which the substituent on the borane replaces the ligand on the palladium concluding with the reductive elimination of the palladium to form the new carbon-carbon bond. The base may serve as a new labile ligand to palladium or it may activate the borane by coordination.
Generalized reaction,
Oxidative addition and ligand exchange,
Borane activation
Reaction,
(h)
Explanation of Solution
The reaction is given as,
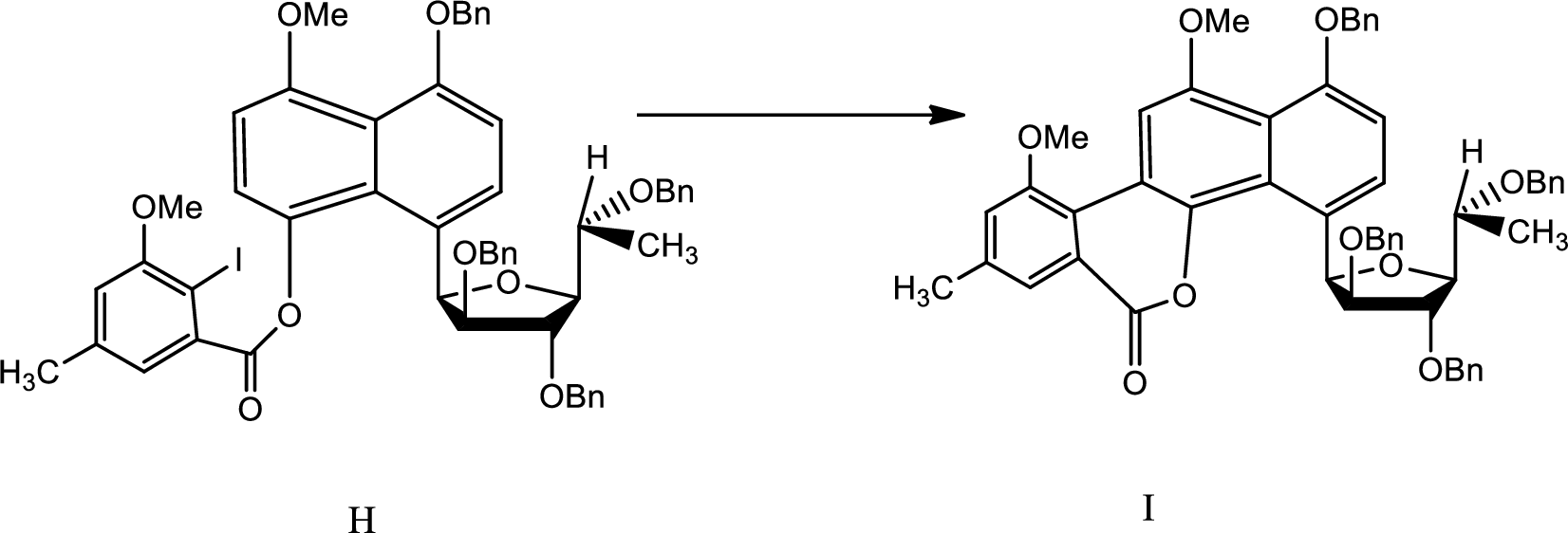
The reaction proceeds as follows,
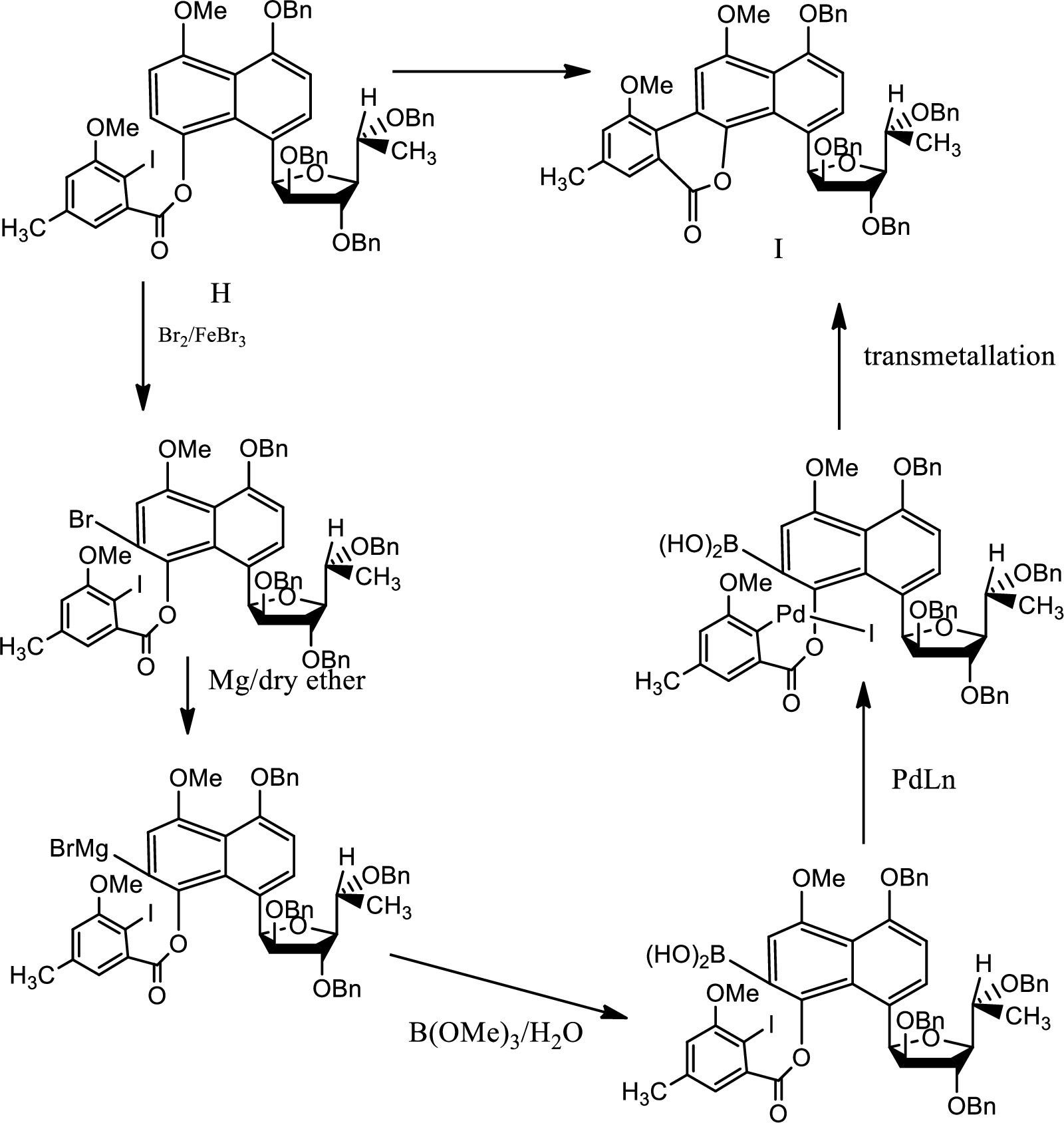
1st boron has to be added so that via transmetallation by intramolecular Suzuki coupling the product can be formed.
(i)
Interpretation:
Reagent needed to form Gilvocarcin M. from I has to be interpreted.
Concept introduction:
Protection-deprotection:
A protecting group is introduced to a molecule by chemical modification of a
Hydrogenolysis:
Hydrogenolysis is a chemical reaction whereby a carbon carbon bond or carbon heteroatom single bond is cleaved by hydrogen gas catalytically.
(i)
Explanation of Solution
The reaction is given as,
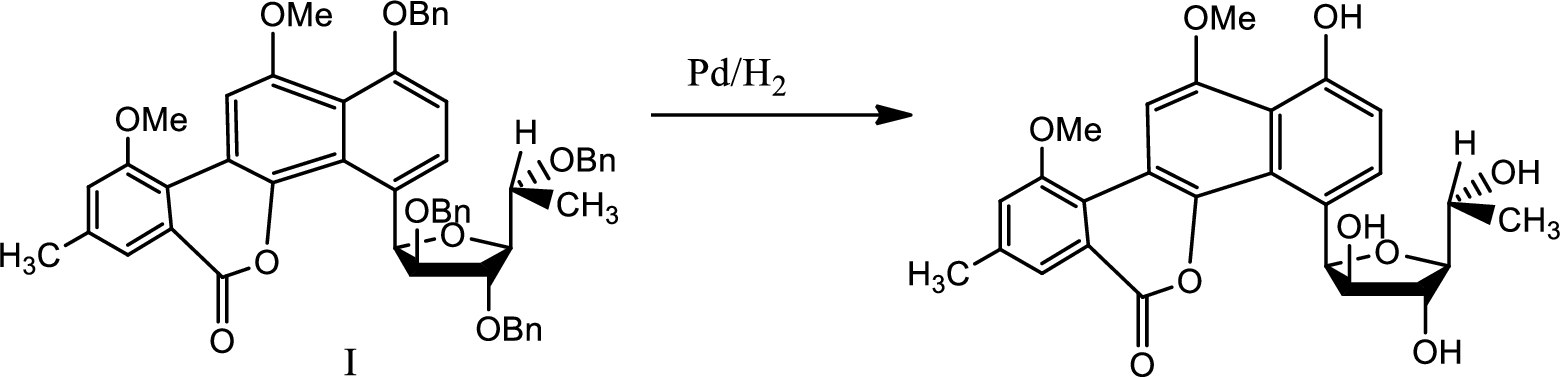
Here deprotection is done by hydrogenolysis with the help of palladium and catalytic amount of hydrogen.
(j)
Interpretation:
Probable source of chiral centres has to be found.
Concept introduction:
Chiral centre:
Chiral centre is defined as an atom bonded to four different chemical species. It is a stereo centre that holds the atom in such way that the structure may not be superimposable to its mirror image. They give optical isomerism.
(j)
Explanation of Solution
The source of the chiral centre is only the five membered carbohydrate ring attached to the benzene ring as shown below,
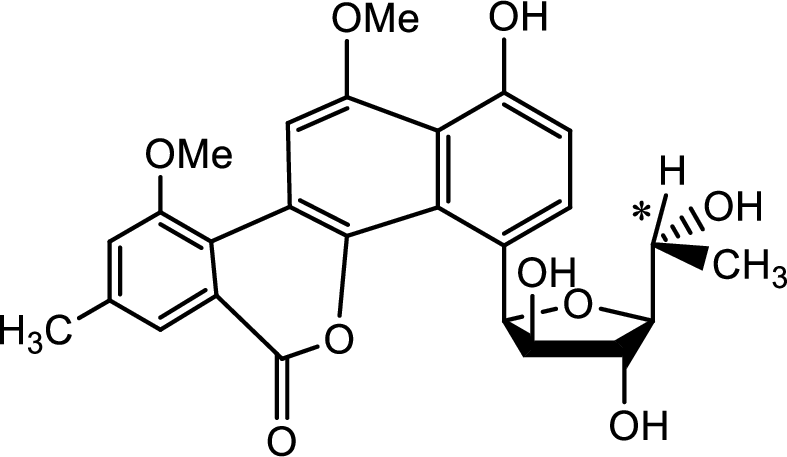
The chiral centre is marked with the asterisk.
(k)
Interpretation:
The need of protection of hydroxyl group has to be stated.
Concept introduction:
Protection-deprotection:
A protecting group is introduced to a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction specially in multistep organic synthesis.
(k)
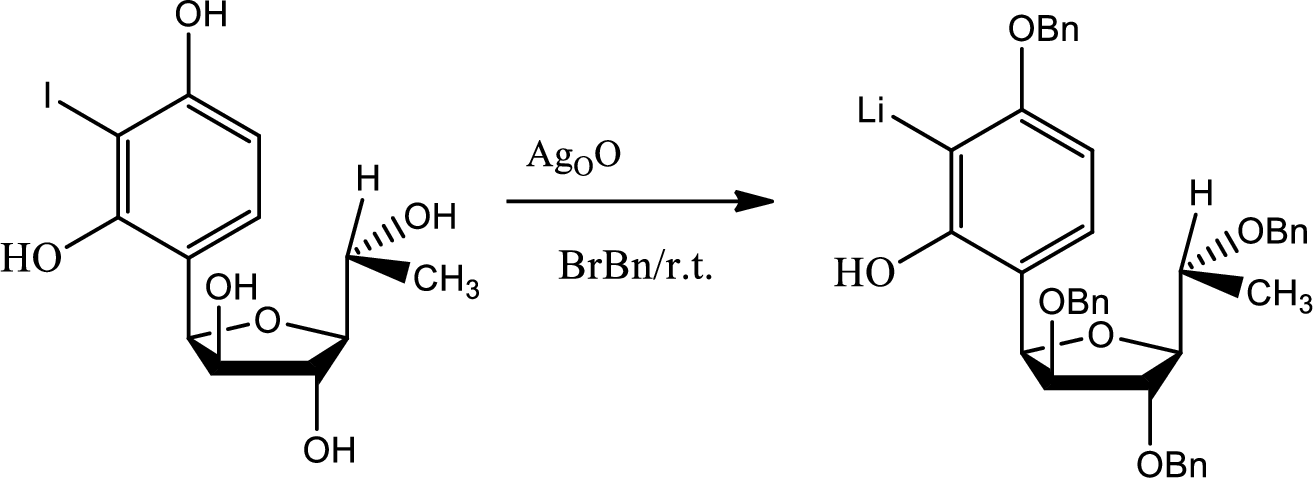
Explanation of Solution
If protection has not been done to the hydroxyl groups then there were possibilities of getting so many unwanted products.
During the acid base reaction all the hydroxyl groups could have lost the proton and thus all the substitution could have taken place in different places. The benzyne formation could have hampered and the stereochemistry could have changed.
The process of protection to the hydroxyl group is given by,
Want to see more full solutions like this?
Chapter 24 Solutions
Organic Chemistry, Loose-leaf Version
- Can u show the process as to how to get these?arrow_forwardSketch the expected 'H NMR spectra for the following compound. Label all of the H's in the structure and the corresponding signal for the spectra you sketch. Make sure you include the integration value and the splitting pattern for each signal Indicate how many signals you would expect in the 13C NMRarrow_forwardUse IUPAC naming rules to name the following hydrocarbon compounds: CH2-CH3 | a) CH-CH-CH2-CH-CH-CH3 b) | CH2 CH3 | CH3 CH3 \ / C=C H 1 H CH2-CH3 c) d) CH=C-CH3 e) CH3-CH2-CH2-CH=CH-CH3 f) CH2=CH-CH2-CH=CH-CH3 g) CH3-CH2-C = C-CH2-CH3 h)arrow_forward
- Q5 Name the following : a. b. C. d. e.arrow_forward25. Predict the major product of the following reaction. 1 equivalent of each of the starting materials was used. H₂C CH3 CH3 H3C H3C H3C. CH2 + H3C. heat CH3 CH H.C. CH3 H.C H.C CH3 CH CH3 CH3 A B C Earrow_forwardFind chemical structures based on the below information. a) Chemical formula C6H8O Compound is aromatic plus has two 1H NMR peaks that integrated for 3 each that are singlets (it could have more peaks in the 1H NMR b) Chemical Formula: C6H100 Compounds is conjugated 'H NMR has a signal that integrates for 6 and is a doublet IR spectra has a signal at 1730 cm-1arrow_forward
- Jaslev Propose a synthesis of the following starting from benzene and any other reagents and chemicals. No mechanisms are required. Indicate the condition for each step plus the major product for each step. More than two steps are required. Step 1 Step 2 مہد Brarrow_forwardPart C: The line formula for another branched alkane is shown below. i. In the IUPAC system what is the root or base name of this compound? ii. How many alkyl substituents are attached to the longest chain? iii. Give the IUPAC name for this compound.arrow_forwardPart D: Draw the Structural Formula for 4-ethyl-2-methylhexane Part E. Draw the Structural Formula for 1-chloro-3,3-diethylpentane (Chloro = Cl)arrow_forward
- Part B: The line formula for a branched alkane is shown below. a. What is the molecular formula of this compound? Number of C. Number of H b. How many carbon atoms are in the longest chain? c. How many alkyl substituents are attached to this chain?arrow_forward24. What is the major product for the following reaction? Mg J. H.C CH H,C- Then H₂O OH Br C HO E HO H.C CH H.C- CH₂ CH₂ All of these are possiblearrow_forwardstructures. Explain why the major product(s) are formed over the minor product(s) using the Draw the major and product and the complete mechanism for all products with all resonance mechanism/resonance structures of the major and minor products in your explanation. HONO2 H2SO4arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


