
(a)
Interpretation:
The types of
Concept introduction:
Functional groups:
The functional group is defined as an atom or group of atoms combined in a specific manner that gives the chemical properties of the organic compound and they are the main reason for the chemical reactivity of the compound. Compounds having similar functional groups undergoes similar type of reactions.
(b)
Interpretation:
The number of chiral centres present in estrone that has to be calculated.
Concept introduction:
Chiral centre:
Chiral centre is defined as an atom bonded to four different chemical species. It is a stereo centre that holds the atom in such way that the structure may not be superimposable to its mirror image. They give optical isomerism.
(c)
Interpretation:
Structural formula for the compounds
Concept introduction:
Structural formula:
The structural formula of a compound can be defined as a graphic representation of the molecular structure showing how the atoms are arranged and the
Retrosynthetic approach:
Retrosynthetic analysis is a technique for planning a synthesis mainly for complex organic molecules where the complex target molecule is reduced into a sequence of progressively simpler structures along a pathway which ultimately leads to the identification of a simple starting molecule or easily available from which the synthesis can be developed.
During retrosynthetic analysis the target molecule is symmetrically broken down by a combination of functional group interconversion and disconnection. This disconnection is related to breaking of carbon-carbon bond of a molecule to generate simpler fragments. The complete set of disconnections and functional group interconversions for a specified target molecule is what constitutes a retrosynthetic plan.
Heck reaction:
The Heck reaction is the
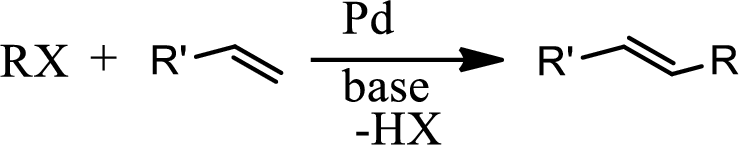
(d)
Interpretation:
The pathway of converting compounds
Concept introduction:
Heck reaction:
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene in presence of a base and palladium catalyst to form a substituted alkene. The reaction is as follows,
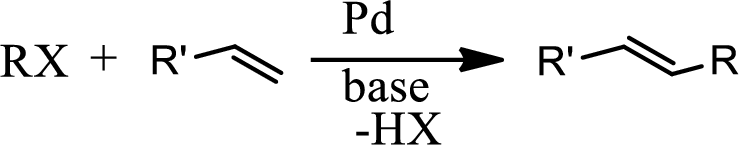
(e)
Interpretation:
The stereochemistry of compound
Concept introduction:
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene in presence of a base and palladium catalyst to form a substituted alkene. The reaction is as follows,
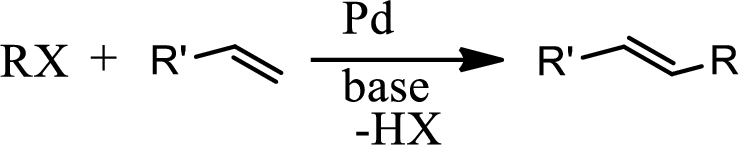
(f)
Interpretation:
The pathway of conversion of tertiary butyl ether to acetone to form estrone from compound
Concept introduction:
Oxidation of secondary alcohol:
Oxidation of alcohol to carbonyl is very difficult as the reaction is so fast that it always ends up by giving acid. Hence for secondary alcohol to get oxidised to carbonyl selectively PCC is used.
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Organic Chemistry, Loose-leaf Version
- What is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.arrow_forwardWhat is the missing intermediate 1 and the final product 2. Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediate and how it occurs and how the final product is former.arrow_forwardWhat would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forward
- For benzene, the ∆H° of vaporization is 30.72 kJ/mol and the ∆S° of vaporization is 86.97 J/mol・K. At 1.00 atm and 228.0 K, what is the ∆G° of vaporization for benzene, in kJ/mol?arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forward
- Which of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forwardWhat is the major product of the following reaction? O O OH OH 1. BH 2. H₂O₂, NaOH OH OHarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

