
Interpretation:
The proper reactant and the proper coupling reaction pathway have to be suggested for the given
Concept Introduction:
Cross coupling:
A cross coupling reaction is defined as a reaction that creates a
In the case of palladium catalysed cross-coupling reactions the other metal or metalloids are commonly
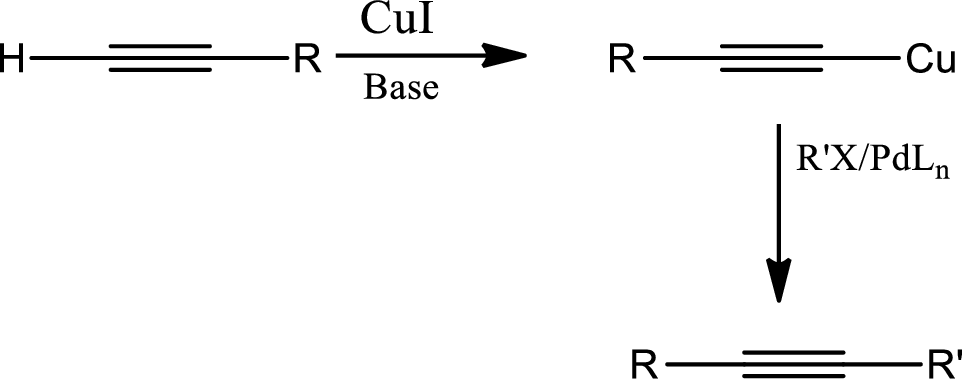
Sonogashira coupling:
The
Generalized reaction,
Suzuki coupling:
The Suzuki coupling uses a boron compound and an alkenyl, aryl or alkyl halide or triflate as the carbon sources with a palladium salt as a catalyst. The reaction is mainly used to form biaryls. The mechanism of the reaction starts with an oxidative addition followed by transmetallation in which the substituent on the borane replaces the ligand on the palladium concluding with the reductive elimination of the palladium to form the new carbon-carbon bond. The base may serve as a new labile ligand to palladium or it may activate the borane by coordination.
Generalized reaction,
Oxidative addition and ligand exchange,
Borane activation
Reaction,
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Organic Chemistry, Loose-leaf Version
- Can I get help on drawing my arrowsarrow_forwardCan I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forward
- Please explain how to calculate the pH.arrow_forwardI'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


