
Concept explainers
a)
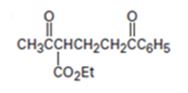
Interpretation:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are to be given.
Concept introduction:
Michael reaction involves the conjugate addition of a stable enolate ion derived from a β-ketoesters or β-diketones or β-ketonitriles or malonic esters (donors) to an unhindered α,β-unsaturated
To give:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown.
Answer to Problem 62AP
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are
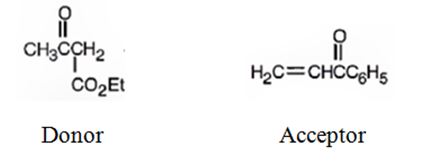
Explanation of Solution
An analysis of the structure of the compound indicates that it is formed by the reaction between the ethylacetoacetate (nucleophilic donor) and phenyl vinyl ketone (electrophilic acceptor).
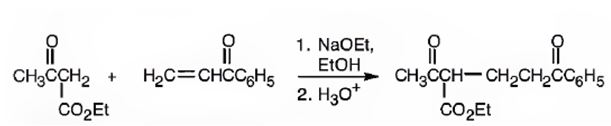
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are
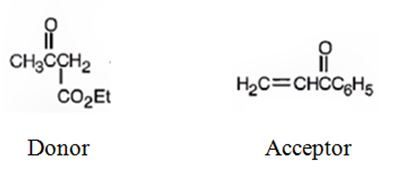
b)
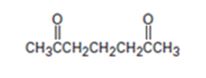
Interpretation:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are to be given.
Concept introduction:
Michael reaction involves the conjugate addition of a stable enolate ion derived from a β-ketoesters or β-diketones or β-ketonitriles or malonic esters (donors) to an unhindered α,β-unsaturated ketones or aldehydes or esters or thioesters or nitriles or amides or nitro compounds (acceptors). The enolate ion from the donor attacks the double bond in acceptor. A new bond is formed between the α-carbon of the donor and the β-carbon of the unsaturated ester.
To give:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown.
Answer to Problem 62AP
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

Explanation of Solution
An analysis of the structure of the compound indicates that it is formed by the reaction between the ethylacetoacetate (nucleophilic donor) and methyl vinyl ketone (electrophilic acceptor).
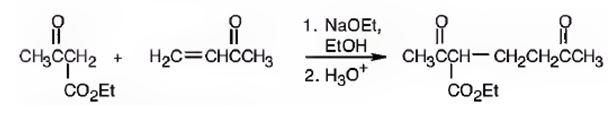
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

c)
Interpretation:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are to be given.
Concept introduction:
Michael reaction involves the conjugate addition of a stable enolate ion derived from a β-ketoesters or β-diketones or β-ketonitriles or malonic esters (donors) to an unhindered α,β-unsaturated ketones or aldehydes or esters or thioesters or nitriles or amides or nitro compounds (acceptors). The enolate ion from the donor attacks the double bond in acceptor. A new bond is formed between the α-carbon of the donor and the β-carbon of the unsaturated ester.
To give:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown.
Answer to Problem 62AP
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

Explanation of Solution
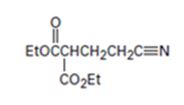
An analysis of the structure of the compound indicates that it is formed by the reaction between the ethylacetoacetate (nucleophilic donor) and vinyl nitrile (electrophilic acceptor).
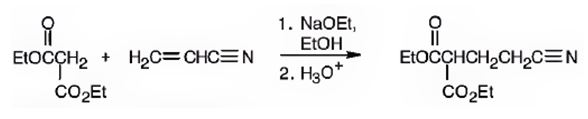
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

d)
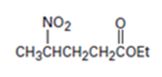
Interpretation:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are to be given.
Concept introduction:
Michael reaction involves the conjugate addition of a stable enolate ion derived from a β-ketoesters or β-diketones or β-ketonitriles or malonic esters (donors) to an unhindered α,β-unsaturated ketones or aldehydes or esters or thioesters or nitriles or amides or nitro compounds (acceptors). The enolate ion from the donor attacks the double bond in acceptor. A new bond is formed between the α-carbon of the donor and the β-carbon of the unsaturated ester.
To give:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown.
Answer to Problem 62AP
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

Explanation of Solution
An analysis of the structure of the compound indicates that it is formed by the reaction between nitro ethane (nucleophilic donor) and ethylacrylate (electrophilic acceptor).
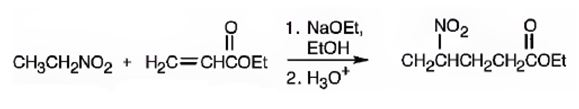
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are
e)
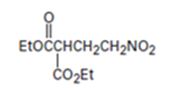
Interpretation:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound are to be given.
Concept introduction:
Michael reaction involves the conjugate addition of a stable enolate ion derived from a β-ketoesters or β-diketones or β-ketonitriles or malonic esters (donors) to an unhindered α,β-unsaturated ketones or aldehydes or esters or thioesters or nitriles or amides or nitro compounds (acceptors). The enolate ion from the donor attacks the double bond in acceptor. A new bond is formed between the α-carbon of the donor and the β-carbon of the unsaturated ester.
To give:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown.
Answer to Problem 62AP
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

Explanation of Solution
An analysis of the structure of the compound indicates that it is formed by the reaction between the ethylsuccinate (nucleophilic donor) and nitro ethene (electrophilic acceptor).
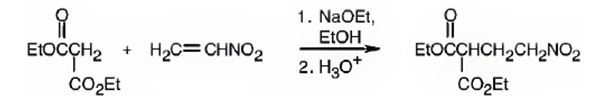
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

f)
Interpretation:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are to be given.
Concept introduction:
Michael reaction involves the conjugate addition of a stable enolate ion derived from a β-ketoesters or β-diketones or β-ketonitriles or malonic esters (donors) to an unhindered α,β-unsaturated ketones or aldehydes or esters or thioesters or nitriles or amides or nitro compounds (acceptors). The enolate ion from the donor attacks the double bond in acceptor. A new bond is formed between the α-carbon of the donor and the β-carbon of the unsaturated ester.
To give:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown.
Answer to Problem 62AP
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are
Explanation of Solution
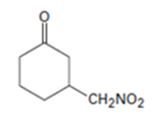
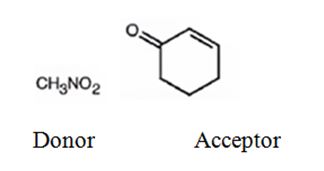
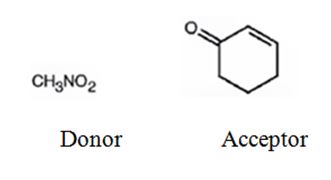
An analysis of the structure of the compound indicates that it is formed by the reaction between nitro methane (nucleophilic donor) and 2-cyclopentenone (electrophilic acceptor).
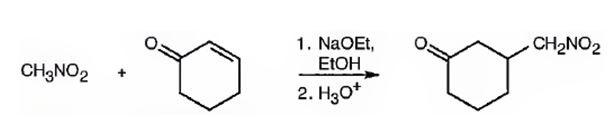
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are
Want to see more full solutions like this?
Chapter 23 Solutions
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
- Help me understand this! Thank you in advance.arrow_forward22.22 For each compound, indicate which group on the ring is more strongly activating and then draw a structural formula of the major product formed by nitration of the compound. Br CHO (a) CH3 (b) (c) CHO CH3 SO₂H (d) ☑ OCHS NO₂ (e) (f) CO₂H NHCOCH3 NHCOCH, (h) CHS 22.23 The following molecules each contain two aromatic rings. (b) 000-100- H3C (a) (c) Which ring in each undergoes electrophilic aromatic substitution more readily? Draw the major product formed on nitration.arrow_forwardV Consider this step in a radical reaction: Br: ? What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. ⚫ionization termination initialization neutralization none of the abc Explanation Check 80 Ο F3 F1 F2 2 F4 01 % do5 $ 94 #3 X 5 C MacBook Air 25 F5 F6 66 ©2025 ˇ F7 29 & 7 8arrow_forward
- Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardno aiarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts.arrow_forward
- 8:44 PM Sun Apr 13 Earn Freecash.com O Measurement and Matter =1 Setting up a unit conversion 110 Eddie says... ✰ www-awu.aleks.com A student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation. Note: your answer should be in the form of one or more fractions multiplied together. (- 4 J kJ -7.0 × 10 ☐ = ? mmol.°C mol °C x10 μ Explanation Check □·□ torox.io Grey Hill LLC. All Rightsarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts please.arrow_forwardi need help with the folarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


