
a)
Interpretation:
Which aldehyde or
Concept introduction:
To state:
Which aldehyde or ketone gives in an aldol reaction the compound represented by the model.
Answer to Problem 23VC
The ketone that gives in an aldol reaction the compound represented by the model is 3-pentanone.
Explanation of Solution
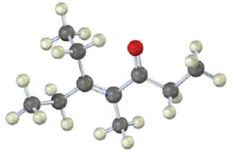
The compound represented by the model is
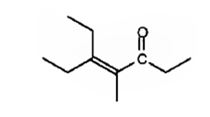
An analysis of the structure indicates it can be produced by the aldol reaction between two 3-pentanone molecules and dehydrating the aldol obtained.
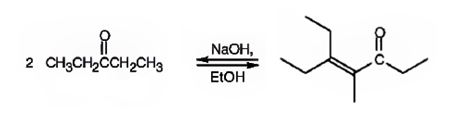
The ketone that gives in an aldol reaction the compound represented by the model is 3-pentanone.
b)
Interpretation:
Which aldehyde or ketone gives in an aldol reaction the compound represented by the model is to be stated.
Concept introduction:
Aldehydes with α-hydrogen undergo a base catalyzed carbonyl condensation reaction in aldol condensation. In this reaction two molecules of the reactant combine by forming a bond between α- carbon of one molecule and the carbonyl carbon of the second molecule. The product obtained is a β-hydroxyaldehyde.
To state:
Which aldehyde or ketone gives in an aldol reaction the compound represented by the model.
Answer to Problem 23VC
The aldehyde that gives in an aldol reaction the compound represented by the model is 3-methylbutanal.
Explanation of Solution
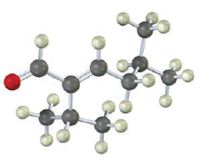
The compound represented by the model is
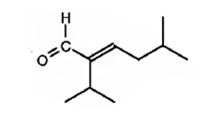
An analysis of the structure indicates it can be produced by the aldol reaction between two 3-methylbutanal molecules and dehydrating the aldol obtained.
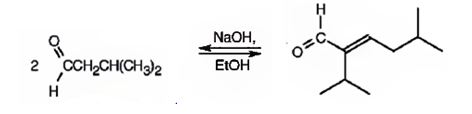
The aldehyde that gives in an aldol reaction, the compound represented by the model is 3-methylbutanal.
Want to see more full solutions like this?
Chapter 23 Solutions
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
- Can I get help on drawing my arrowsarrow_forwardCan I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forward
- Please explain how to calculate the pH.arrow_forwardI'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

