
Concept explainers
a)
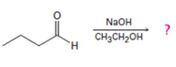
Interpretation:
The dehydration product formed in the addition reaction of butanal along with the mechanism of its formation is to be predicted.
Concept introduction:
The β- hydroxyl aldehyde or ketone obtained loses a molecule of water upon heating to give an α, β- unsaturated aldehyde or ketone.
To predict:
The dehydration product formed in the addition reaction of butanal along with the mechanism of its formation.
Answer to Problem 28MP
The dehydration product formed in the addition reaction of butanal is 2-ethyl-2-hexenal.
The mechanism of its formation is
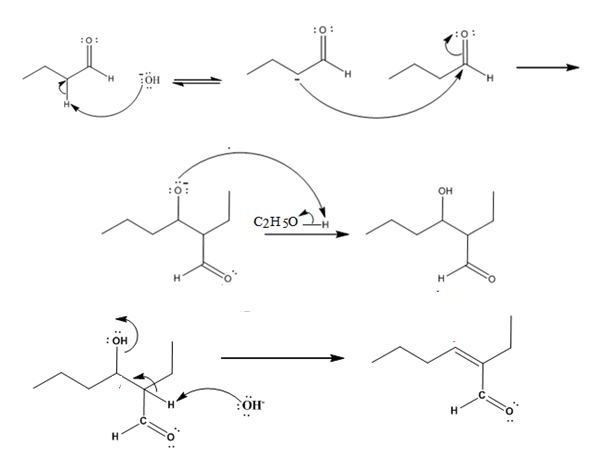
Explanation of Solution
In the first step the base picks up an acidic proton from the diketone to produce the enolate anion. In the next step the nucleophilic enolate anion attacks the electrophilic carbon of the other carbonyl group in the same molecule to give an alkoxide. In the next step the alkoxide intermediate is protonated to yield the β- hydroxyl aldehyde. Removal of a proton by the base from the hydroxy aldehyde leads to the formation of α, β- unsaturated aldehyde.
The dehydration product formed in the addition reaction of butanal is 2-ethyl-2-hexenal.
The mechanism of its formation is

b)
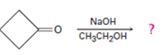
Interpretation:
The dehydration product formed in the addition reaction given along with the mechanism of its formation is to be predicted.
Concept introduction:
Aldehydes and ketones that have α- hydrogen atom undergo aldol condensation to yield a β- hydroxyl aldehyde or ketone as the product. The reaction occurs in three steps i) Abstraction of α- hydrogen by a base to yield an enolate anion ii) Attack of the anion on the carbonyl carbon of another molecule iii) Protonation of the alkoxide intermediate.
The β- hydroxyl aldehyde or ketone obtained on heating loses a water molecule to yield an α, β- unsaturated aldehyde or ketone.
To identify:
The dehydration product formed in the addition reaction of cyclobutanone along with the mechanism of its formation.
Answer to Problem 28MP
The dehydration product formed in the addition reaction of cyclobutanone is
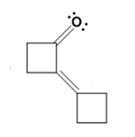
The mechanism of its formation is
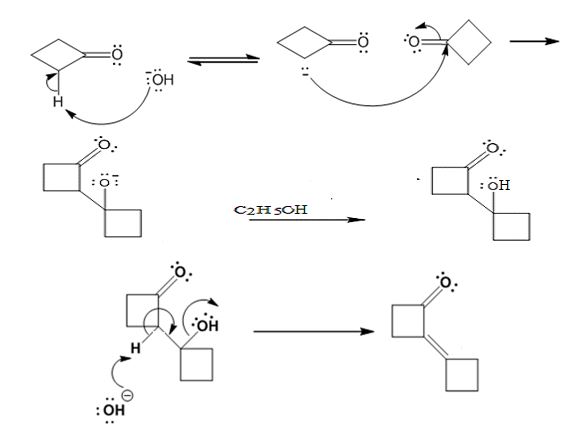
Explanation of Solution
In the first step the base picks up a proton from the α-carbon of one cyclobutanone molecule to produce the enolate anion. In the next step the nucleophilic enolate anion attacks the electrophilic carbonyl carbon of another cyclobutanoe molecule to give an alkoxide. In the next step the alkoxide intermediate is protonated to yield a β- hydroxyketone. In the final step the base picks up a proton from the hydroxyl group that leads to the formation of α, β- unsaturated ketone.
The dehydration product formed in the addition reaction of cyclobutanone is

The mechanism of its formation is

c)
Interpretation:
The dehydration product formed in the addition reaction given along with the mechanism of its formation is to be predicted.
Concept introduction:
The reaction given is a mixed aldol reaction. Aldehydes and ketones that have α- hydrogen atom undergo aldol condensation to yield a β- hydroxyl aldehyde or ketone as the product. The reaction occurs in three steps i) Abstraction of α- hydrogen by a base to yield an enolate anion ii) Attack of the anion on the carbonyl carbon of another molecule iii) Protonation of the alkoxide intermediate.
The β- hydroxyl aldehyde or ketone loses a molecule of water when heated in the presence of a base to yield α, β- unsaturated aldehyde or ketone.
To identify:
The dehydration product formed in the addition reaction given along with the mechanism of its formation.
Answer to Problem 28MP
The dehydration product formed in the addition reaction given is
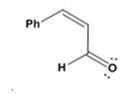
The mechanism of its formation is
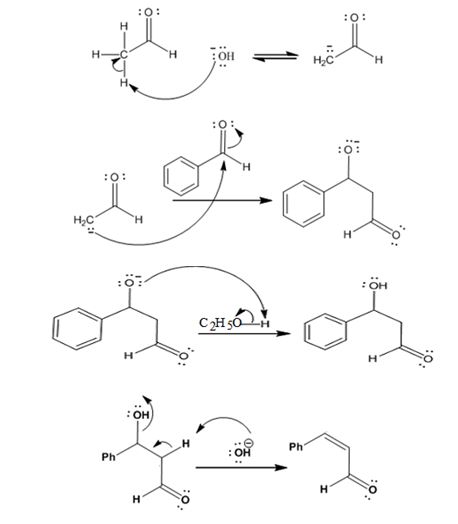
Explanation of Solution
In the first step the base picks up a proton from the α-carbon of acetaldehyde as it has α- hydrogen atoms to produce the enolate anion. In the next step the nucleophilic enolate anion attacks the electrophilic carbonyl carbon of benzaldehyde to give an alkoxide. In the next step the alkoxide intermediate is protonated to yield a β- hydroxyaldehyde. In the final step the base picks up a proton from the hydroxyl group that leads to the formation of α, β- unsaturated aldehyde.
The dehydration product formed in the addition reaction given is

The mechanism of its formation is

d)
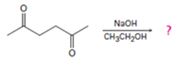
Interpretation:
The product formed in the addition reaction given along with the mechanism of its formation is to be predicted.
Concept introduction:
In intramolecular aldol reactions dicarbonyl compounds such as diketones react with a base to yield a cyclic enone as the products. The reaction occurs in four steps i) Abstraction of α-hydrogen by a base to yield an enolate anion ii) Attack of the anion on the carbonyl carbon in another molecule iii) Protonation of the alkoxide intermediate. iv) Loss of water from the keto alcohol upon heating.
To identify:
The product formed in the addition reaction given along with the mechanism of its formation.
Answer to Problem 28MP
The product formed in the addition reaction given is
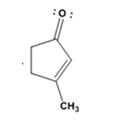
The mechanism of its formation is
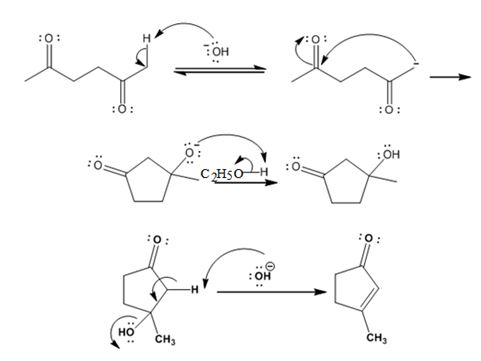
Explanation of Solution
In the first step the base picks up an acidic proton from the diketone to produce the enolate anion. In the next step the nucleophilic enolate anion attacks the electrophilic carbon of the other carbonyl group in the same molecule to give an alkoxide. In the next step the alkoxide intermediate is protonated to yield a hydroxyl ketone. In the final step the base picks up a proton from the hydroxyl group that leads to the formation of α, β- unsaturated aldehyde.
The product formed in the addition reaction given is

The mechanism of its formation is

Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry
- Identify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forward
- Rank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forward
- Why isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forward
- Complete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forwardQ Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forward
