
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23, Problem 31P
Draw the products of the following reaction, where T is Tritium:
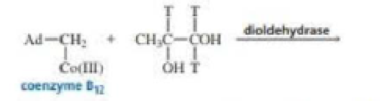
(Hint: Tritium is 3H, a hydrogen atom with two neutrons. Although a C — T bond breaks more slowly than a C — H bond, it is still the first bond in the substrate that breaks.)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Nail polish remover containing acetone was spilled in a room 5.23 m × 3.28 m × 2.76 m.
Measurements indicated that 2,250 mg of acetone evaporated. Calculate the acetone concentration in micrograms per cubic meter.
Please help me answer number 1. 1. If your graphs revealed a mathematical relationship between specific heat and atomic mass, write down an equation for the relationship.
I also don't understand, is the equation from the line regression the one that I'm suppose use to show the relationship? If so could you work it all the way out?
Describe the principle of resonance and give a set of Lewis Structures to illustrate your explanation.
Chapter 23 Solutions
EBK ORGANIC CHEMISTRY
Ch. 23.1 - Prob. 2PCh. 23.1 - Prob. 3PCh. 23.2 - How many conjugated double bonds are there in a....Ch. 23.2 - Instead of adding to the 4a position and...Ch. 23.2 - Prob. 7PCh. 23.3 - Prob. 8PCh. 23.3 - Acetolactate synthase is another TPP-requiring...Ch. 23.3 - Acetolactate synthase transfers the acyl group of...Ch. 23.3 - Prob. 12PCh. 23.5 - Which compound is more easily decarboxylated?
Ch. 23.5 - Prob. 14PCh. 23.5 - Explain why the ability of PLP to catalyze an...Ch. 23.5 - Explain why the ability of PLP to catalyze an...Ch. 23.5 - The enzyme that catalyzes the C C bond cleavage...Ch. 23.5 - Propose a mechanism for the ,-elimination reaction...Ch. 23.6 - Ethanolamine ammonia lyase, a coenzyme...Ch. 23.6 - Prob. 20PCh. 23.7 - How do the structure of tetrahydrofolate and...Ch. 23.7 - What is the source of the methyl group in...Ch. 23.8 - Thiols such as ethanethiol and propanethiol can be...Ch. 23 - How does the metal ion in carboxypeptidase A...Ch. 23 - Prob. 24PCh. 23 - Prob. 25PCh. 23 - For each of the following reactions, name both the...Ch. 23 - Prob. 27PCh. 23 - When transaminated, the three branched-chain amino...Ch. 23 - What acyl groups have we seen transferred by...Ch. 23 - Propose a mechanism for the following reaction:Ch. 23 - Draw the products of the following reaction, where...Ch. 23 - When UMP is dissolved in T2O, exchange of T for H...Ch. 23 - Dehydratase is a PLP-requiring enzyme that...Ch. 23 - In addition to the reaction mentioned in Section...Ch. 23 - PLP can catalyze both ,-elimination reactions...Ch. 23 - The glycine cleavage system is a group of four...Ch. 23 - Prob. 37PCh. 23 - FADH2 reduces , -unsaturated thioesters to...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used hand raitingarrow_forwardIt is not unexpected that the methoxyl substituent on a cyclohexane ring prefers to adopt the equatorial conformation. OMe H A G₂ = +0.6 kcal/mol OMe What is unexpected is that the closely related 2-methoxytetrahydropyran prefers the axial conformation: H H OMe OMe A Gp=-0.6 kcal/mol Methoxy: CH3O group Please be specific and clearly write the reason why this is observed. This effect that provides stabilization of the axial OCH 3 group in this molecule is called the anomeric effect. [Recall in the way of example, the staggered conformer of ethane is more stable than eclipsed owing to bonding MO interacting with anti-bonding MO...]arrow_forward206 Pb 82 Express your answers as integers. Enter your answers separated by a comma. ▸ View Available Hint(s) VAΣ ΜΕ ΑΣΦ Np, N₁ = 82,126 Submit Previous Answers ? protons, neutronsarrow_forward
- Please draw the inverted chair forms of the products for the two equilibrium reactions shown below. Circle the equilibrium reaction that would have a AG = 0, i.e., the relative energy of the reactant (to the left of the equilibrium arrows) equals the relative energy of the product? [No requirement to show or do calculations.] CH3 CH3 HH CH3 1 -CH3arrow_forward5. Please consider the Newman projection of tartaric acid drawn below as an eclipsed conformer (1). Please draw the most stable conformer and two intermediate energy conformers noting that staggered conformers are lower in energy than eclipsed forms even if the staggered conformers have gauche relationships between groups. [Draw the substituents H and OH on the front carbons and H, OH and CO₂H on the back carbons based on staggered forms. -CO₂H is larger than -OH.] OH COH ICOOH COOH COOH 1 2 COOH COOH 3 4 Staggered Staggered Staggered (most stable) Indicate the number of each conformer above (1, 2, 3 and 4) that corresponds to the relative energies below. Ref=0 Rotation 6. (60 points) a. Are compounds 1 and 2 below enantiomers, diastereomers or identical? OH OH HO HO LOH HO HO OH 2 OH OH b. Please complete the zig-zag conformation of the compound (3R,4S)-3,4-dichloro-2,5-dimethylhexane by writing the respective atoms in the boxes. 3.arrow_forwardThe plutonium isotope with 144 neutrons Enter the chemical symbol of the isotope.arrow_forward
- The mass ratio of sodium to fluorine in sodium fluoride is 1.21:1. A sample of sodium fluoride produced 26.1 gg of sodium upon decomposition. How much fluorine was formed?arrow_forward32S 16 Enter your answers numerically separated by a comma. Np. Nn = 跖 ΟΙ ΑΣΦ Submit Request Answer ? protons, neutronsarrow_forward2. Which dimethylcyclohexane compounds shown below exhibit symmetry and therefore are not chiral and would not rotate plane polarized light. 1 CH3 CH CH3 CH3 2 3 CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License