
Concept explainers
(a)
Interpretation:
The electron-dot structure of the given structure is to be drawn and described.

Concept introduction:
The electron dot structures can be described as the structure in which electrons are represented around the atoms in the molecule.
In the resonance structure, the electrons are moved towards the more electronegative atoms and more positive charges. The arrow will be originated from pi electrons or unshared pair of electrons and move towards the more electronegative atoms and positive charges.
(b)
Interpretation:
The electron-dot structure of the given structure is to be drawn and described.
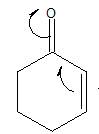
Concept introduction:
The electron dot structures can be described as the structure in which electrons are represented around the atoms in the molecule.
In the resonance structure, the electrons are moved towards the more electronegative atoms and more positive charges. The arrow will be originated from pi electrons or unshared pair of electrons and move towards the more electronegative atoms and positive charges.
(c)
Interpretation:
The electron-dot structure of the given structure is to be drawn and described.
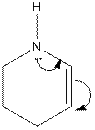
Concept introduction:
The electron dot structures can be described as the structure in which electrons are represented around the atoms in the molecule.
In the resonance structure, the electrons are moved towards the more electronegative atoms and more positive charges. The arrow will be originated from pi electrons or unshared pair of electrons and move towards the more electronegative atoms and positive charges.
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
CHEMISTRY-MASTERINGCHEMISTRY W/ETEXT
- How many chiral centers are there in the following molecule? HO 0 1 ○ 2 ♡ 4 'N'arrow_forwardThe following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forward
- Given the following data, determine the order of the reaction with respect to H2. H2(g) + 21Cl(g) → I2(g) + 2HCl(g) Experiment [H2] (torr) [ICI] (torr) Rate (M/s) 1 250 325 0.266 2 250 81 0.0665 3 50 325 0.266arrow_forwardWhich one of the following molecules is chiral? H- NH₂ H3C དང་།་ OH H HO H₂N HO- -H CHO -OH H HO- OH H- -H CH₂OH OHarrow_forwardThe structure of an unsaturated phospholipid is shown below. Which region of the molecule is most hydrophilic ? H₂N-CH₂ H₂C IV CH3 CH3 hydro-water philic-likes = Hydrophilic likes water ○ IV All regions are equally hydrophilic. IIIarrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





