
Concept explainers
(a)
Interpretation:
The line drawing nitrogen containing compound is to be drawn and formal charge on each atom is to be shown.
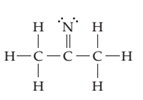
Concept introduction:
The line drawing is a chemical structure in which carbon and hydrogen molecules are not drawn. In these types of structures, lines are used to draw structure representing molecules. In the electron dot structure, the lone pair of electrons is represented as two dots on the respective symbol of the atom.
Formal charge is the charge present on each atom in the molecule; it is calculated by the following formula:
Formal Charge = Number of valence electrons on
(b)
Interpretation:
The line drawing containing compound is to drawn and formal charge on each atom is to be shown.
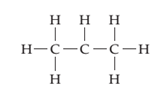
Concept introduction:
The line drawing is a chemical structure in which carbon and hydrogen molecules are not drawn. In these types of structures, lines are used to draw structure representing molecules. In the electron dot structure, the lone pair of electrons is represented as two dots on the respective symbol of the atom.
Formal charge is the charge present on each atom in the molecule; it is calculated by the following formula:
Formal Charge = Number of valence electrons on atom − [Number of non-bonded electrons + Number of bonds].
(c)
Interpretation:
The line drawing nitrogen containing compound is to drawn and formal charge on each atom is to be shown.
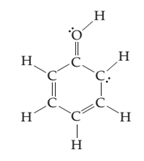
Concept introduction:
The line drawing is a chemical structure in which carbon and hydrogen molecules are not drawn. In these types of structures, lines are used to draw structure representing molecules. In the electron dot structure, the lone pair of electrons is represented as two dots on the respective symbol of the atom.
Formal charge is the charge present on each atom in the molecule; it is calculated by the following formula:
Formal Charge = Number of valence electrons on atom − [Number of non-bonded electrons + Number of bonds].
Trending nowThis is a popular solution!

Chapter 23 Solutions
CHEMISTRY-MASTERINGCHEMISTRY W/ETEXT
- Construct a molecular orbital energy-level diagram for BeH2. Sketch the MO pictures (schematic representation) for the HOMO and LUMO of BeH2 [Orbital Potential Energies, H (1s): -13.6 eV; Be (2s): -9.3 eV, Be (2p): -6.0 eV]arrow_forwardIndicate the isomers of the A(H2O)6Cl3 complex. State the type of isomerism they exhibit and explain it briefly.arrow_forwardState the formula of the compound potassium μ-dihydroxydicobaltate (III) tetraoxalate.arrow_forward
- Consider the reaction of the cyclopentanone derivative shown below. i) NaOCH2CH3 CH3CH2OH, 25°C ii) CH3!arrow_forwardWhat constitutes a 'reference material', and why does its utilization play a critical role in the chemical analysis of food products? Provide examples.arrow_forwardExplain what calibration is and why it is essential in relation to food analysis. Provide examples.arrow_forward
- The cobalt mu-hydroxide complex cobaltate(III) of potassium is a dinuclear complex. Correct?arrow_forwardThe cobalt mi-hydroxide complex cobaltate(III) of potassium is a dinuclear complex. Correct?arrow_forward3. Arrange the different acids in Exercise B # 2 from the strongest (1) to the weakest acid (10). 1. 2. (strongest) 3. 4. 5. 6. 7. 8. 9. 10 10. (weakest)arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning





