
Concept explainers
(a)
Interpretation:
The classification of the carbohydrate by indicating the nature of the carbonyl group and the number of carbon present on it is to be done.
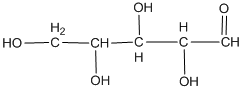
Concept introduction:
The major classifications of carbohydrate on the basis of the nature of the carbonyl group are:
- Aldose
- Ketose
If the carbohydrate has an
The naming of carbohydrates on the basis of the number of carbon present in it is done by the method used in the case of hydrocarbon. For example, prop for three followed by −ose as suffix.
(b)
Interpretation:
The classification of the carbohydrate by indicating the nature of the carbonyl group and the number of carbon present on it is to be done.
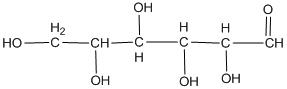
Concept introduction:
The major classifications of carbohydrate on the basis of the nature of the carbonyl group are:
- Aldose
- Ketose
If the carbohydrate has an aldehyde group present on it, then it is called aldose and the presence of a ketone group makes it ketose.
The naming of carbohydrates on the basis of the number of carbon present in it is done by the method used in the case of hydrocarbon. For example: - prop for three followed by −ose as suffix.
(c)
Interpretation:
The classification of the carbohydrate by indicating the nature of the carbonyl group and the number of carbon present on it is to be done.
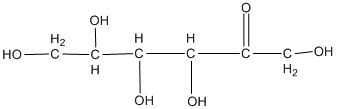
Concept introduction:
The major classifications of carbohydrate on the basis of the nature of the carbonyl group are:
- Aldose
- Ketose
If the carbohydrate has an aldehyde group present on it, then it is called aldose and the presence of a ketone group makes it ketose.
The naming of carbohydrates on the basis of the number of carbon present in it is done by the method used in the case of hydrocarbon. For example, prop for three followed by −ose as suffix.
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
CHEMISTRY-MASTERINGCHEMISTRY W/ETEXT
- Consider the reaction of the cyclopentanone derivative shown below. i) NaOCH2CH3 CH3CH2OH, 25°C ii) CH3!arrow_forwardWhat constitutes a 'reference material', and why does its utilization play a critical role in the chemical analysis of food products? Provide examples.arrow_forwardExplain what calibration is and why it is essential in relation to food analysis. Provide examples.arrow_forward
- The cobalt mu-hydroxide complex cobaltate(III) of potassium is a dinuclear complex. Correct?arrow_forwardThe cobalt mi-hydroxide complex cobaltate(III) of potassium is a dinuclear complex. Correct?arrow_forward3. Arrange the different acids in Exercise B # 2 from the strongest (1) to the weakest acid (10). 1. 2. (strongest) 3. 4. 5. 6. 7. 8. 9. 10 10. (weakest)arrow_forward
- Name Section Score Date EXERCISE B pH, pOH, pка, AND PKD CALCULATIONS 1. Complete the following table. Solution [H+] [OH-] PH РОН Nature of Solution A 2 x 10-8 M B 1 x 10-7 M C D 12.3 6.8 2. The following table contains the names, formulas, ka or pka for some common acids. Fill in the blanks in the table. (17 Points) Acid Name Formula Dissociation reaction Ka pka Phosphoric acid H₂PO₁ H3PO4 H++ H₂PO 7.08 x 10-3 Dihydrogen H₂PO H₂PO H+ HPO 6.31 x 10-6 phosphate Hydrogen HPO₁ 12.4 phosphate Carbonic acid H2CO3 Hydrogen HCO 6.35 10.3 carbonate or bicarbonate Acetic acid CH,COOH 4.76 Lactic acid CH₂CHOH- COOH 1.38 x 10 Ammonium NH 5.63 x 10-10 Phenol CH₂OH 1 x 10-10 Protonated form CH3NH3* 3.16 x 10-11 of methylaminearrow_forwardIndicate whether it is true that Co(III) complexes are very stable.arrow_forwardMnO2 acts as an oxidant in the chlorine synthesis reaction.arrow_forward
- In Potassium mu-dihydroxydicobaltate (III) tetraoxalate K4[Co2(C2O4)4(OH)2], indicate whether the OH ligand type is bidentate.arrow_forwardImagine an electrochemical cell based on these two half reactions with electrolyte concentrations as given below: Oxidation: Pb(s) → Pb2+(aq, 0.10 M) + 2 e– Reduction: MnO4–(aq, 1.50 M) + 4 H+(aq, 2.0 M) + 3 e– → MnO2(s) + 2 H2O(l) Calculate Ecell (assuming temperature is standard 25 °C).arrow_forward: ☐ + Draw the Fischer projection of the most common naturally-occurring form of aspartate, with the acid group at the top and the side chain at the bottom. Important: be sure your structure shows the molecule as it would exist at physiological pH. Click and drag to start drawing a structure. ✓arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div  ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





