
Concept explainers
(a)
Interpretation:
The possibility of resonance structure of the given structure is to be discussed.
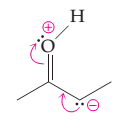
Concept introduction:
The resonance or mesmeric effect is the phenomena of delocalization of (-bonds in the chain and ring.
(b)
Interpretation:
The possibility of resonance structure of the given structure is to be discussed.
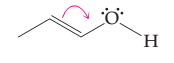
Concept introduction:
The resonance or mesmeric effect is the phenomena of delocalization of (-bonds in the chain and ring.
(c)
Interpretation:
The possibility of resonance structure of the given structure is to be discussed.
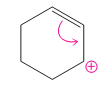
Concept introduction:
The resonance or mesmeric effect is the phenomena of delocalization of (-bonds in the chain and ring.
(d)
Interpretation:
The possibility of resonance structure of the given structure is to be discussed.
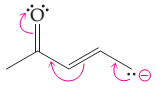
Concept introduction:
The resonance or mesomeric effect is the phenomena of delocalization of (-bonds in the chain and ring.
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
CHEMISTRY-MASTERINGCHEMISTRY W/ETEXT
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





