
Concept explainers
(a)
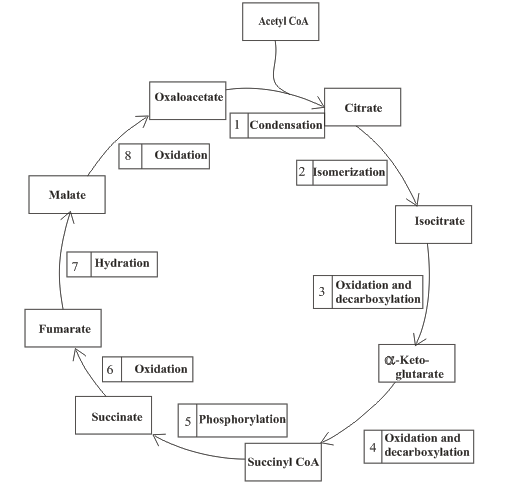
Interpretation: To determine the general type of reaction in the citric acid cycle that involves malate as a reactant.
Concept introduction: Citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
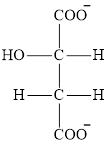
The structure of malate ion that is involved in the citric acid cycle is:
(b)
Interpretation: To determine the general type of reaction in the citric acid cycle that involves malate as a product.
Concept introduction: Citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
An overview of the citric acid cycle is as follows:

The structure of malate ion that is involved in the citric acid cycle is:

(c)
Interpretation: To determine the general type of reaction in the citric acid cycle that involves isocitrate as a reactant.
Concept introduction: Citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
An overview of the citric acid cycle is as follows:

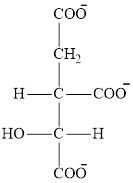
The structure of isocitrate ion that is involved in the citric acid cycle is:
(d)
Interpretation: To determine the general type of reaction in the citric acid cycle that involves isocitrate as a product.
Concept introduction: Citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
An overview of the citric acid cycle is as follows:

The structure of isocitrate ion that is involved in the citric acid cycle is:

Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
- Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forwardIndicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.arrow_forward
- helparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning


