
a)
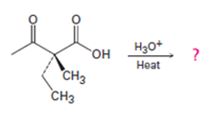
Interpretation:
Whether the
Concept introduction:
The decarboxylation of a β- keto acid takes place through the formation of a planar enol intermediate which then tautomerizes to the keto form. If the β- keto acid is optically active and if the chiral centre is involved in the reaction, the ketone product will be racemic and optically inactive. If the chiral centre is not involved in the reaction then the optical activity is retained in the ketone product.
To state:
Whether the ketone obtained by the decarboxylation of the optically active β- keto acid will be optically active or not.
To propose:
A mechanism to explain the formation of the ketone.
b)
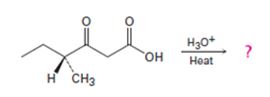
Interpretation:
Whether the ketone obtained by the decarboxylation of the optically active β- keto acid will be optically active or not is to be stated. A mechanism to explain the formation of the ketone is to be proposed.
Concept introduction:
The decarboxylation of a β- keto acid takes place through the formation of a planar enol intermediate which then tautomerizes to the keto form. If the β- keto acid is optically active and if the chiral centre is involved in the reaction, the ketone product will be racemic and optically inactive. If the chiral centre is not involved in the reaction then the optical activity is retained in the ketone product.
To state:
Whether the ketone obtained by the decarboxylation of the optically active β- keto acid will be optically active or not.
To propose:
A mechanism to explain the formation of the ketone.
Trending nowThis is a popular solution!

Chapter 22 Solutions
EBK ORGANIC CHEMISTRY
- When anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forwardDraw the major product in each of the following reaction:arrow_forward
- Draw the mechanism for the following Friedel-Craft reaction. AlBr3 Brarrow_forward(a) Draw the structures of A and B in the following reaction. (i) NaNH2, NH3(1) A + B (ii) H3O+arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Consider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forwardIn a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


