
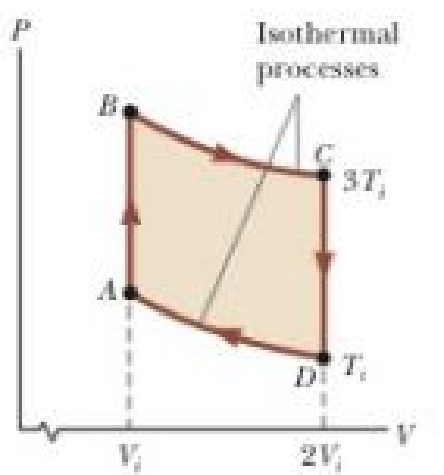
In 1816, Robert Stirling, a Scottish clergyman, patented the Stirling engine, which has found a wide variety of applications ever since, including current use in solar energy collectors to transform sunlight into electricity. Fuel is burned externally to warm one of the engine’s two cylinders. A fixed quantity of inert gas moves cyclically between the cylinders, expanding in the hot one and contracting in the cold one. Figure P21.33 represents a model for its thermodynamic cycle. Consider n moles of an ideal monatomic gas being taken once through the cycle, consisting of two isothermal processes at temperatures 3Ti and Ti and two constant-volume processes. Let us find the efficiency of this engine. (a) Find the energy transferred by heat into the gas during the isovolumetric process AB. (b) Find the energy transferred by heat into the gas during the isothermal process BC. (c) Find the energy transferred by heat into the gas during the isovolumetric process CD. (d) Find the energy transferred by heat into the gas during the isothermal process DA. (e) Identify which of the results from parts (a) through (d) are positive and evaluate the energy input to the engine by heat. (f) From the first law of
Trending nowThis is a popular solution!

Chapter 22 Solutions
Physics for Scientists and Engineers With Modern Physics
Additional Science Textbook Solutions
Applications and Investigations in Earth Science (9th Edition)
Fundamentals of Anatomy & Physiology (11th Edition)
Biology: Life on Earth (11th Edition)
HUMAN ANATOMY
Cosmic Perspective Fundamentals
- Which of the following best describes how to calculate the average acceleration of any object? Average acceleration is always halfway between the initial acceleration of an object and its final acceleration. Average acceleration is always equal to the change in velocity of an object divided by the time interval. Average acceleration is always equal to the displacement of an object divided by the time interval. Average acceleration is always equal to the change in speed of an object divided by the time interval.arrow_forwardThe figure shows the velocity versus time graph for a car driving on a straight road. Which of the following best describes the acceleration of the car? v (m/s) t(s) The acceleration of the car is negative and decreasing. The acceleration of the car is constant. The acceleration of the car is positive and increasing. The acceleration of the car is positive and decreasing. The acceleration of the car is negative and increasing.arrow_forwardWhich figure could represent the velocity versus time graph of a motorcycle whose speed is increasing? v (m/s) v (m/s) t(s) t(s)arrow_forward
- Unlike speed, velocity is a the statement? Poisition. Direction. Vector. Scalar. quantity. Which one of the following completesarrow_forwardNo chatgpt pls will upvote Already got wrong chatgpt answerarrow_forward3.63 • Leaping the River II. A physics professor did daredevil stunts in his spare time. His last stunt was an attempt to jump across a river on a motorcycle (Fig. P3.63). The takeoff ramp was inclined at 53.0°, the river was 40.0 m wide, and the far bank was 15.0 m lower than the top of the ramp. The river itself was 100 m below the ramp. Ignore air resistance. (a) What should his speed have been at the top of the ramp to have just made it to the edge of the far bank? (b) If his speed was only half the value found in part (a), where did he land? Figure P3.63 53.0° 100 m 40.0 m→ 15.0 marrow_forward
 Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning





