
Concept explainers
(a)
Interpretation:
Whether the statement “The most common primary protein structures are the
Concept introduction:
Proteins are the
Answer to Problem 60E
The statement “The most common primary protein structures are the
Explanation of Solution
Primary structure of a protein is straight chain polymer of amino acids linked by the amide linkages. The
The statement “The most common primary protein structures are the
(b)
Interpretation:
Whether the statement “Sulfur-sulfur bonds called disulfide linkages are important in the tertiary protein structure of many proteins” is true or false is to be stated.
Concept introduction:
Proteins are the polymers of amino acids also known as polypeptides. They are formed by the peptide bonding between two acids formed by the elimination of the water molecule between the carboxylic acid and amine group leading to the formation of the amide group. Various structure or forms of the proteins are found in the body.
Answer to Problem 60E
The statement “Sulfur-sulfur bonds called disulfide linkages are important in the tertiary protein structure of many proteins” is a true statement.
Explanation of Solution
The tertiary structure of the proteins is curling of the secondary protein structure on itself. The stability of the tertiary structure of many tertiary proteins is enhanced by the sulfur-sulfur linkages between the sulfur-containing amino acids, for example, Cysteine.
Therefore, the statement “Sulfur-sulfur bonds called disulfide linkages are important in the tertiary protein structure of many proteins” is true.
The statement “Sulfur-sulfur bonds called disulfide linkages are important in the tertiary protein structure of many proteins” is a true statement.
(c)
Interpretation:
Whether the statement “An enzyme substrate is the product of an enzyme-catalyzed reaction” is true or false is to be stated.
Concept introduction:
Enzymes are the biochemical catalysts present in the human body or livings organism to catalyst their biochemical process. Enzymes fasten up the
Answer to Problem 60E
The statement “An enzyme substrate is the product of an enzyme-catalyzed reaction” is a false statement.
Explanation of Solution
The substrate in an enzyme-catalyzed reaction is the reactant reacting with the enzyme to give a back product. Substrate attaches to the active site of the enzyme for the catalysis. Therefore, the statement “An enzyme substrate is the product of an enzyme-catalyzed reaction” is a false statement.
The statement “An enzyme substrate is the product of an enzyme-catalyzed reaction” is a false statement.
(d)
Interpretation:
Whether the statement “The catalytic properties of an enzyme exist at its active site” is true or false is to be stated.
Concept introduction:
Enzymes are the biochemical catalysts present in the human body or livings organism to catalyst their biochemical process. Enzymes fasten up the rate of reaction by a very large factor. Substrate comes to the active site of the enzyme for the catalysis.
Answer to Problem 60E
The statement “The catalytic properties of an enzyme exist at its active site” is a true statement.
Explanation of Solution
The substrate in an enzyme-catalyzed reaction is the reactant reacting with the enzyme to give a back product. Substrate attaches to the active site of the enzyme for the catalysis. Therefore, the statement “The catalytic properties of an enzyme exist at its active site” is true.
The statement “The catalytic properties of an enzyme exist at its active site” is a true statement.
(e)
Interpretation:
Whether the statement “Carbohydrates are the hydrates of carbon; that is, compounds that consist of carbon and water molecules” is true or false is to be stated.
Concept introduction:
Sugar molecules are examples of carbohydrates. Sugars are the
Answer to Problem 60E
The statement “Carbohydrates are the hydrates of carbon; that is, compounds that consist of carbon and water molecules” is a false statement.
Explanation of Solution
Carbohydrates are thought to be “hydrates of carbon” as suggested by the name but they are classified as the aldehydes or ketones containing two or more hydroxide
The statement “Carbohydrates are the hydrates of carbon; that is, compounds that consist of carbon and water molecules” is a false statement.
(f)
Interpretation:
Whether the statement “Glucose is also called blood sugar” is true or false is to be stated.
Concept introduction:
Glucose is a sugar molecule also known as carbohydrates. It is a six carbon aldose sugar which means it contains aldehyde and hydroxide groups. Glucose has two forms
Answer to Problem 60E
The statement “Glucose is also called blood sugar” is a true statement.
Explanation of Solution
Glucose is a sugar molecule also known as carbohydrates. It is a six carbon aldose sugar which means it contains aldehyde and hydroxide groups. Glucose has two forms
The statement “Glucose is also called blood sugar” is a true statement.
(g)
Interpretation:
Whether the statement “Sucrose is a glucose-fructose combination” is true or false is to be stated.
Concept introduction:
Carbohydrates are classified as the compounds that are aldehydes or ketone containing two or more
Answer to Problem 60E
The statement “Sucrose is a glucose-fructose combination” is a true statement.
Explanation of Solution
The disaccharide is the combination of two monosaccharides may be identical or different. Sucrose is one of the most famous examples of disaccharides. It is the combination of two monosaccharide units glucose and fructose. Therefore, the statement “Sucrose is a glucose-fructose combination” is true.
The statement “Sucrose is a glucose-fructose combination” is a true statement.
(h)
Interpretation:
Whether the statement “Fats and oils are distinguished by their
Concept introduction:
Lipids are the water-insoluble compounds found in the living organisms. Lipids are parted into three classes (1) Fats, oils and phospholipids, (2) waxes, and (3) steroids.
All these compounds are insoluble in polar solvents like water because of the large presence of hydrophobic groups in them.
Answer to Problem 60E
The statement “Fats and oils are distinguished by their state of matter at room temperature” is a true statement.
Explanation of Solution
Fats and oils are the triesters of long-chain carboxylic acids known as fatty acids. The ester is formed from simple alcohol glycerol. The fats and oils are also referred to as triacylglycerols. The fats at room temperature are solid while the oils are liquid at room temperature. Therefore, the statement “Fats and oils are distinguished by their state of matter at room temperature” is true.
The statement “Fats and oils are distinguished by their state of matter at room temperature” is a true statement.
(i)
Interpretation:
Whether the statement “Steroids have three cyclopentane rings and one cyclohexane ring fused” is true or false is to be stated.
Concept introduction:
Lipids are the water-insoluble compounds found in the living organisms. Lipids are parted into three classes (1) Fats, oils and phospholipids, (2) waxes, and (3) steroids.
All these compounds are insoluble in polar solvents like water because of the large presence of hydrophobic groups in them.
Answer to Problem 60E
The statement “Steroids have three cyclopentane rings and one cyclohexane ring fused” is a false statement.
Explanation of Solution
Steroids contain a four ring fused system. Three are six carbon and one is five carbon ring. Six carbon ring is known as cyclohexane and five carbon ring is known as cyclopentane. Therefore, the statement “Steroids have three cyclopentane rings and one cyclohexane ring fused” is false.
The structure of one of the steroids cholesterol is shown below.
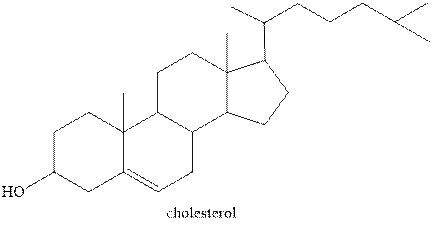
Figure 1
The statement “Steroids have three cyclopentane rings and one cyclohexane ring fused” is a false statement.
(j)
Interpretation:
Whether the statement “Nucleic acid monomers are called nucleotides” is true or false is to be stated.
Concept introduction:
Nucleotides are the monomers of
Answer to Problem 60E
The statement “Nucleic acid monomers are called nucleotides” is a true statement.
Explanation of Solution
Nucleic acids are the polymer of nucleotides. Nucleotides are phosphate and base-sugar combination. Nucleotides contain two types of sugars in them one is ribose sugar and other is deoxyribose sugar.
Therefore, the statement “Nucleic acid monomers are called nucleotides” is true.
The statement “Nucleic acid monomers are called nucleotides” is a true statement.
(k)
Interpretation:
Whether the statement “DNA is a nucleotide (phosphate-sugar-base) polymer” is true or false is to be stated.
Concept introduction:
Nucleotides are the monomers of nucleic acids. DNA and RNA are examples of nucleotides. Nucleotides consist of three parts (a) cyclic nitrogenous base (b) a sugar (ribose or deoxyribose sugar) (c) phosphate group.
Answer to Problem 60E
The statement “DNA is a nucleotide (phosphate-sugar-base) polymer” is a true statement.
Explanation of Solution
Nucleic acids are nucleotide polymers. Nucleotides are a combination of phosphate and base-sugar. DNA is one of the examples of nucleic acids. DNA contains nucleotides having deoxyribose sugar and specific bases (adenine, guanine, thymine, and cytosine).
Therefore, the statement “DNA is a nucleotide (phosphate-sugar-base) polymer” is true.
The statement “DNA is a nucleotide (phosphate-sugar-base) polymer” is a true statement.
Want to see more full solutions like this?
Chapter 22 Solutions
Bundle: Introductory Chemistry: An Active Learning Approach, 6th + OWLv2, 1 term (6 months) Printed Access Card
- Draw the mechanism (including all curved arrows for electron movement) showing how the maleicanhydride is attacked by the anthracene and formation of the final Diels Alder product.arrow_forwardProvide the missing information. *see imagearrow_forwardProvide the missing information. *see imagearrow_forward
- Provide the missing information. *see imagearrow_forwardI have a bottle of butanal that has been improperly used by lab workers. They allowed a traceamount NaOH (aq) to contaminate the bottle. What is now in my bottle of “butanal? What is the molecular name and functional group name? Draw the structure.arrow_forwardProvide the missing information. *see imagearrow_forward
- First image: Why can't the molecule C be formed in those conditions Second image: Synthesis for lactone C its not an examarrow_forwardFirst image: I have to show the mecanism for the reaction on the left, where the alcohol A is added fast in one portion Second image: I have to show the mecanism of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primaryarrow_forwardFirst image: I have to explain why the molecule C is never formed in those conditions. Second image: I have to propose a synthesis for the lactone Aarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





