
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 22, Problem 58QE
(a)
Interpretation Introduction
Interpretation:
The chiral carbons have to be located in the following molecule.
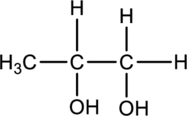
Concept Introduction:
The molecules whose mirror-image structures cannot be superimposed on the original structure are said to be chiral molecules.
A carbon atom is said to be Chiral when it is bonded to four different substituents. Chiral carbon atoms are known as asymmetric centers.
(b)
Interpretation Introduction
Interpretation:
The chiral carbons have to be located in the following molecule.
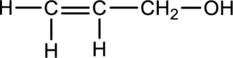
Concept Introduction:
Refer part (a).
(c)
Interpretation Introduction
Interpretation:
The chiral carbons have to be located in the following molecule.
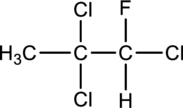
Concept Introduction:
Refer part (a).
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore
inorganic byproducts.
Incorrect, 5 attempts remaining
1. NaOCH3/CH3OH
2. Acidic workup
Select to Draw
O
Incorrect, 5 attempts remaining
The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or
intermediate structures and recount the number of carbon atoms in the parent chain of your structure.
OK
Using a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40.
a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L)
b) Calculate the molar absorptivity, a, of the 1645 cm-1 band
c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c
d) Calculate x for the 1645 cm-1 band
Convert 1.38 eV into wavelength (nm) and wavenumber (cm-1) (c = 2.998 x 108 m/s; h = 6.626 x 10-34 J*s).
Chapter 22 Solutions
Chemistry: Principles and Practice
Ch. 22 - Prob. 1QECh. 22 -
Explain what is special about the element carbon...Ch. 22 - Prob. 3QECh. 22 - Prob. 4QECh. 22 - Prob. 5QECh. 22 - Prob. 6QECh. 22 - Prob. 7QECh. 22 - Prob. 8QECh. 22 - Prob. 9QECh. 22 - Prob. 10QE
Ch. 22 - Prob. 11QECh. 22 - Prob. 12QECh. 22 - Prob. 13QECh. 22 - Prob. 14QECh. 22 - Prob. 15QECh. 22 - Prob. 16QECh. 22 - Prob. 17QECh. 22 - Prob. 18QECh. 22 - Prob. 19QECh. 22 - Prob. 20QECh. 22 - Prob. 21QECh. 22 - Prob. 22QECh. 22 - Prob. 23QECh. 22 - Prob. 24QECh. 22 - Prob. 25QECh. 22 - Prob. 26QECh. 22 - Prob. 27QECh. 22 - Prob. 28QECh. 22 - Prob. 29QECh. 22 - Prob. 30QECh. 22 - Prob. 31QECh. 22 - Prob. 32QECh. 22 - Prob. 33QECh. 22 - Prob. 34QECh. 22 - Prob. 35QECh. 22 - Prob. 36QECh. 22 - Prob. 37QECh. 22 - Prob. 38QECh. 22 - Prob. 39QECh. 22 - Prob. 40QECh. 22 - Prob. 41QECh. 22 - Prob. 42QECh. 22 - Prob. 43QECh. 22 - Prob. 44QECh. 22 - Prob. 45QECh. 22 - Prob. 46QECh. 22 - Prob. 47QECh. 22 - Prob. 48QECh. 22 - Prob. 49QECh. 22 - Prob. 50QECh. 22 - Prob. 51QECh. 22 - Prob. 52QECh. 22 - Prob. 53QECh. 22 - Prob. 54QECh. 22 - Name the following compounds. (a) FCH2CH2CH2OH (b)...Ch. 22 - Prob. 56QECh. 22 - Prob. 57QECh. 22 - Prob. 58QECh. 22 - Prob. 59QECh. 22 - Prob. 60QECh. 22 - Prob. 61QECh. 22 - Prob. 62QECh. 22 - Prob. 63QECh. 22 - Prob. 64QECh. 22 - Prob. 65QECh. 22 - Prob. 66QECh. 22 - Prob. 67QECh. 22 - Prob. 68QECh. 22 - Prob. 69QECh. 22 - Prob. 70QECh. 22 - Prob. 71QECh. 22 - Prob. 72QECh. 22 - Prob. 73QECh. 22 - Prob. 74QECh. 22 - Prob. 75QECh. 22 - Prob. 76QECh. 22 - Prob. 77QECh. 22 - Prob. 78QECh. 22 - Prob. 79QECh. 22 - Prob. 80QECh. 22 - Prob. 81QECh. 22 - Prob. 82QECh. 22 - Prob. 83QECh. 22 - Prob. 84QECh. 22 - Prob. 85QECh. 22 - Prob. 86QECh. 22 - Prob. 87QECh. 22 - Prob. 88QE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can you help me understand the CBC method on metal bridging by looking at this problem?arrow_forwardA partir de Aluminio y Co(NO3)2ꞏ6H2O, indicar las reacciones a realizar para obtener Azul de Thenard (Al2CoO4).arrow_forwardTo obtain Thenard Blue (Al2CoO4), the following reaction is correct (performed in an oven):Al(OH)3 + Co(OH)2 → Al2CoO4 + 4 H2Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY