
Physics for Scientists and Engineers
10th Edition
ISBN: 9781337553278
Author: Raymond A. Serway, John W. Jewett
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 21, Problem 9P
If a 35.0% -efficient Carnot
Figure P21.2 Schematic representation of a heat engine.
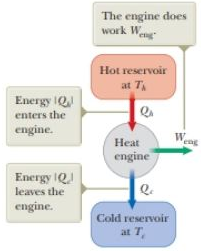
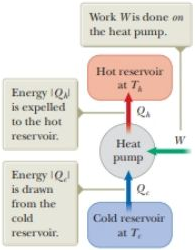
Figure P21.4 Schematic representation of a heat pump.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Is work function of a metals surface related to surface energy and surface tension? What is the need to the work function component in the math of tension of metal surfaces that cannot be provided by existing equations of surface energy and surface tension? What are the key differences in each parameter and variables that allow for a differentiation of each function? What has a more significant meaning work function, surface tension or surface energy? Are there real differences and meaning? Please clarify and if possible provide examples . Does surface tension dependant on thickness of a metal or type of metal surface all having the same thickness? Clearly temperature has a profound change on surface tension what other variables besides temperature are key to surface tension. What if any is there a connection between crystal structure of the element and surface energy and tension? This is NOT a Assignment Question!!!
The cylindrical beam of a 12.7-mW laser is 0.920 cm in diameter. What is the rms value of the electric field?
V/m
Consider a rubber rod that has been rubbed with fur to give the rod a net negative charge, and a glass rod that has been rubbed with silk to give it a net positive charge. After being charged by contact by the fur and silk...?
a. Both rods have less mass
b. the rubber rod has more mass and the glass rod has less mass
c. both rods have more mass
d. the masses of both rods are unchanged
e. the rubber rod has less mass and the glass rod has mroe mass
Chapter 21 Solutions
Physics for Scientists and Engineers
Ch. 21.1 - The energy input to an engine is 4.00 times...Ch. 21.2 - The energy entering an electric heater by...Ch. 21.4 - Three engines operate between reservoirs separated...Ch. 21.6 - (a) Suppose you select four cards at random from a...Ch. 21.7 - An ideal gas is taken from an initial temperature...Ch. 21.7 - True or False: The entropy change in an adiabatic...Ch. 21 - A particular heat engine has a mechanical power...Ch. 21 - The work done by an engine equals one-fourth the...Ch. 21 - Suppose a heat engine is connected to two energy...Ch. 21 - During each cycle, a refrigerator ejects 625 kJ of...
Ch. 21 - A freezer has a coefficient of performance of...Ch. 21 - A heat pump has a coefficient of performance equal...Ch. 21 - One of the most efficient heat engines ever built...Ch. 21 - Why is the following situation impossible? An...Ch. 21 - If a 35.0% -efficient Carnot heat engine (Fig....Ch. 21 - An ideal refrigerator or ideal heat pump is...Ch. 21 - A heat engine is being designed to have a Carnot...Ch. 21 - A power plant operates at a 32.0% efficiency...Ch. 21 - You are working on a summer job at a company that...Ch. 21 - A Carnot heat engine operates between temperatures...Ch. 21 - An electric generating station is designed to have...Ch. 21 - Suppose you build a two-engine device with the...Ch. 21 - A heat pump used for heating shown in Figure...Ch. 21 - A gasoline engine has a compression ratio of 6.00....Ch. 21 - An idealized diesel engine operates in a cycle...Ch. 21 - (a) Prepare a table like Table 21.1 for the...Ch. 21 - Prob. 21PCh. 21 - A Styrofoam cup holding 125 g of hot water at 100C...Ch. 21 - A 1 500-kg car is moving at 20.0 m/s. The driver...Ch. 21 - A 2.00-L container has a center partition that...Ch. 21 - Calculate the change in entropy of 250 g of water...Ch. 21 - What change in entropy occurs when a 27.9-g ice...Ch. 21 - When an aluminum bar is connected between a hot...Ch. 21 - When a metal bar is connected between a hot...Ch. 21 - How fast are you personally making the entropy of...Ch. 21 - Prob. 30APCh. 21 - The energy absorbed by an engine is three times...Ch. 21 - In 1993, the U.S. government instituted a...Ch. 21 - In 1816, Robert Stirling, a Scottish clergyman,...Ch. 21 - Suppose an ideal (Carnot) heat pump could be...Ch. 21 - Review. This problem complements Problem 44 in...Ch. 21 - A firebox is at 750 K, and the ambient temperature...Ch. 21 - A 1.00-mol sample of an ideal monatomic gas is...Ch. 21 - A system consisting of n moles of an ideal gas...Ch. 21 - A heat engine operates between two reservoirs at...Ch. 21 - You are working as an assistant to a physics...Ch. 21 - Prob. 41APCh. 21 - You are working as an expert witness for an...Ch. 21 - An athlete whose mass is 70.0 kg drinks 16.0...Ch. 21 - Prob. 44APCh. 21 - Prob. 45APCh. 21 - A sample consisting of n moles of an ideal gas...Ch. 21 - The compression ratio of an Otto cycle as shown in...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 8. With the aid of a diagram draw the following electric circuit and use the resistor as the load, (a) Closed circuit (b) Open circuitarrow_forwardLab 8 Part 3 PHET Wave Interface simulation. I am having trouble with this part of the lab.arrow_forwardMick and Rick are twins born on Earth in the year 2175. Rick grows up to be an Earth-bound robotics technician while Mick becomes an intergalactic astronaut. Mick leaves the Earth on his first space mission in the year 2200 and travels, according to his clock, for 10 years at a speed of 0.75c. Unfortunately, at this point in his journey, the structure of his ship undergoes mechanical breakdown and the ship explodes. How old is Rick when his brother dies?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY