
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 30P
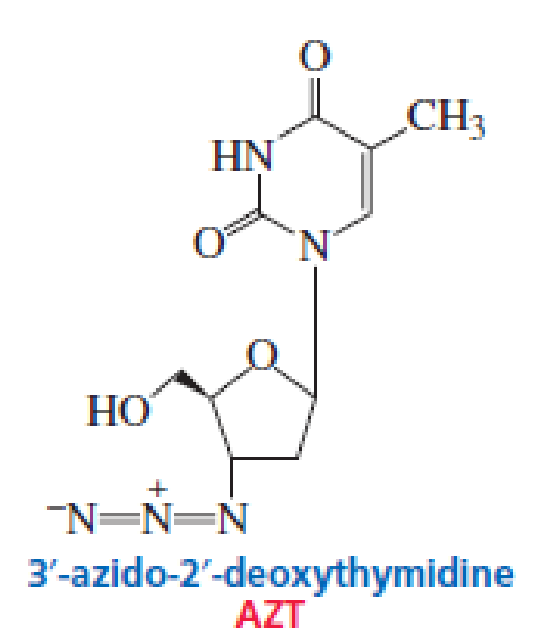
Human immunodeficiency virus (HIV) is the retrovirus that causes AIDS. AZT was one of the first drugs designed to interfere with retroviral DNA synthesis. When cells take up AZT, they convert it to AZT-triphosphate. Explain how AZT interferes with DNA synthesis.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
None
7. Draw a curved arrow mechanism for the following reaction.
HO
cat. HCI
OH
in dioxane
with 4A molecular sieves
Try: Convert the given 3D perspective structure to Newman projection about C2 - C3 bond (C2 carbon in the
front). Also, show Newman projection of other possible staggered conformers and circle the most stable
conformation. Use the template shown.
F
H3C
Br
H
Chapter 21 Solutions
Essential Organic Chemistry, Global Edition
Ch. 21.1 - Draw the structure for each of the following: a....Ch. 21.3 - If one of the strands of DNA has the following...Ch. 21.4 - The 2,3-cyclic phosphodiester that is formed...Ch. 21.5 - Using a dark line for the original parental DNA...Ch. 21.7 - Why do both thymine and uracil specify the...Ch. 21.9 - If methionine is always the first amino acid...Ch. 21.9 - Four Cs occur in a row in the segment of mRNA...Ch. 21.9 - UAA is a stop codon. Why does the UAA sequence in...Ch. 21.9 - A change in which base of a codon would be least...Ch. 21.9 - Write the sequences of bases in the sense strand...
Ch. 21.9 - List the possible codons on mRNA that specify each...Ch. 21.10 - Adenine can be deaminated to hypoxanthine, and...Ch. 21.10 - Explain why thymine cannot be deaminated.Ch. 21.12 - Which of the following base sequences would most...Ch. 21 - Draw structures for the following: a....Ch. 21 - What nonapeptide is coded for by the following...Ch. 21 - What is the sequence of bases in the template...Ch. 21 - What is the sequence of bases in the sense strand...Ch. 21 - What would be the C-terminal amino acid if the...Ch. 21 - What would be the base sequence of the segment of...Ch. 21 - Propose a mechanism for the following reaction:Ch. 21 - A segment of DNA has 18 base pairs. It has 7...Ch. 21 - Match the codon with the anticodon: Codon: AAA GCA...Ch. 21 - Using the single-letter abbreviations for the...Ch. 21 - Indicate whether each functional group of the five...Ch. 21 - Using the A and D designations in Problem 25,...Ch. 21 - Which of the following pairs of dinucleotides are...Ch. 21 - If an mRNA contained only U and G in random...Ch. 21 - Why is the codon a triplet rather than a doublet...Ch. 21 - Human immunodeficiency virus (HIV) is the...Ch. 21 - The amino acid sequences of peptide fragments...Ch. 21 - Which cytosine in the following sense strand of...Ch. 21 - Prob. 33PCh. 21 - Why does DNA not unravel completely before...Ch. 21 - The first amino acid incorporated into a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forward16. Consider the probability distribution p(x) = ax", 0 ≤ x ≤ 1 for a positive integer n. A. Derive an expression for the constant a, to normalize p(x). B. Compute the average (x) as a function of n. C. Compute σ2 = (x²) - (x)², the variance of x, as a function of n.arrow_forward451. Use the diffusion model from lecture that showed the likelihood of mixing occurring in a lattice model with eight lattice sites: Case Left Right A B C Permeable Barrier → and show that with 2V lattice sites on each side of the permeable barrier and a total of 2V white particles and 2V black particles, that perfect de-mixing (all one color on each side of the barrier) becomes increasingly unlikely as V increases.arrow_forward
- 46. Consider an ideal gas that occupies 2.50 dm³ at a pressure of 3.00 bar. If the gas is compressed isothermally at a constant external pressure so that the final volume is 0.500 dm³, calculate the smallest value Rest can have. Calculate the work involved using this value of Rext.arrow_forwardNonearrow_forward2010. Suppose that a 10 kg mass of iron at 20 C is dropped from a heigh of 100 meters. What is the kinetics energy of the mass just before it hits the ground, assuming no air resistance? What is its speed? What would be the final temperature of the mass if all the kinetic energy at impact is transformed into internal energy? The molar heat capacity of iron is Cpp = 25.1J mol-¹ K-1 and the gravitational acceleration constant is 9.8 m s¯² |arrow_forward
- ell last during 7. Write the isotopes and their % abundance of isotopes of i) Cl ii) Br 8. Circle all the molecules that show Molecular ion peak as an odd number? c) NH2CH2CH2NH2 d) C6H5NH2 a) CH³CN b) CH3OHarrow_forwardCalsulate specific heat Dissolution of NaOH ก ง ง Mass of water in cup Final temp. of water + NaOH Initial temp. of water AT Water AH Dissolution NaOH - "CaicuraORT. AH (NaOH)=-AH( 30g (water) 29.0°C 210°C 8°C (82) 100 3.. =1003.20 Conjosarrow_forwardPlease provide throrough analysis to apply into further problems.arrow_forward
- Molecular ion peak: the peak corresponding to the intact morecure (with a positive charge) 4. What would the base peak and Molecular ion peaks when isobutane is subjected to Mass spectrometry? Draw the structures and write the molecular weights of the fragments. 5. Circle most stable cation a) tert-butyl cation b) Isopropyl cation c) Ethyl cation. d)Methyl cationarrow_forwardHow many arrangements are there of 15 indistinguishable lattice gas particles distributed on: a.V = 15 sites b.V = 16 sites c.V = 20 sitesarrow_forwardFor which element is the 3d subshell higher in energy than that 4s subshell? Group of answer choices Zr Ca V Niarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning
Nucleic acids - DNA and RNA structure; Author: MEDSimplified;https://www.youtube.com/watch?v=0lZRAShqft0;License: Standard YouTube License, CC-BY