
Concept explainers
For each amino acid: [1] draw the L enantiomer in a Fischer projection; [2] classify the amino acid as neutral, acidic, or basic; [3) give the three-letter symbol; [4] give the one-letter symbol.
- arginine
- tyrosine
- glutamic acid
- valine
(a)
Interpretation:
The L-isomer of arginine in Fisher projection needs to be drawn and identified as acidic, basic or neutral. The three letter and one symbol for it needs to be determined.
Concept Introduction:
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral center of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
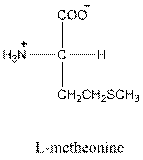
Answer to Problem 26P
- L isomer of arginine is as follows:
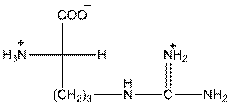
- In arginine, an additional basic nitrogen present. So, it is basic in nature.
- Three-letter symbol for arginine is 'Arg'.
- One letter symbol is 'R'.
Explanation of Solution
Amino acids that have the
To draw the Fisher projection of arginine, place the
at the bottom and to draw L isomer draw
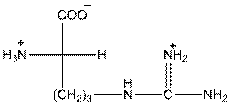
Here, R group is as follows:
In arginine, an additional basic nitrogen present. So, it is basic in nature.
Three-letter symbol for arginine is Arg and one letter symbol is R.
(b)
Interpretation:
The L-isomer of tyrosine in Fisher projection needs to be drawn and identified as acidic, basic or neutral. The three-letter and one symbol for it needs to be determined.
Concept Introduction:
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral centre of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
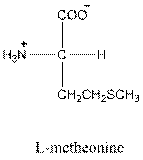
Answer to Problem 26P
- L-isomer of tyrosine is as follows:
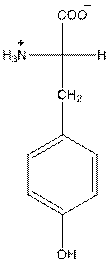
- Tyrosine is neutral in nature.
- Three-letter symbol for tyrosine is 'Tyr'.
- One letter symbol for tyrosine is 'Y'.
Explanation of Solution
Amino acids that have the
To draw the Fisher projection of tyrosine by placing the
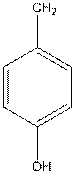
at the bottom and to draw L isomer draw
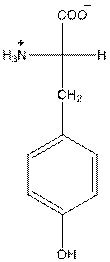
The overall charge of tyrosine is zero as there is one positive and negative charge. Hence, tyrosine is neutral in nature.
Three-letter symbol for tyrosine is 'Tyr' and one letter symbol is 'Y'.
(c)
Interpretation:
The L-isomer of glutamic acid in Fisher projection needs to be drawn and identified as acidic, basic or neutral. The three-letter and one symbol for it needs to be determined.
Concept Introduction:
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral centre of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
Answer to Problem 26P
- L-isomer of glutamic acid is as follows:
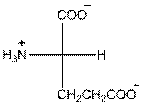
- Glutamic acid is acidic in nature.
- Three-letter symbol for glutamic acid is 'Glu'.
- One letter symbol for glutamic acid is 'E'.
Explanation of Solution
Amino acids that have the
Draw the Fisher projection of glutamic acid by placing the
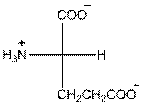
In glutamic acid, one additional acidic carboxyl group present. So, glutamic acid is acidic in nature.
Three-letter symbol for glutamic acid is 'Glu' and one letter symbol for glutamic acid is 'E'.
(d)
Interpretation:
The L-isomer of valine in Fisher projection needs to be drawn and identified as acidic, basic or neutral. The three-letter and one symbol for it needs to be determined.
Concept Introduction:
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral centre of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
Answer to Problem 26P
- L-isomer of valine is as follows:
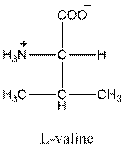
- Valine is neutral in nature.
- Three-letter symbol for valine is 'Val'.
- One letter symbol for valine is 'V'.
Explanation of Solution
Amino acids that have the
Draw the Fisher projection of valine by placing the
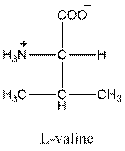
The net charge in valine is zero as there is one positive charge and negative charge in the compound. So, valine is neutral.
Three-letter symbol for valine is 'Val' and one letter symbol for valine is 'V'.
Want to see more full solutions like this?
Chapter 21 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
- Which of the following molecules are NOT typical carbohydrates? For the molecules that are carbohydrates, label them as an aldose or ketose. HO Он ОН ОН Он ОН но ΤΗ HO ОН HO eve Он он ОН ОН ОН If polyethylene has an average molecular weight of 25,000 g/mol, how many repeat units are present?arrow_forwardDraw the a-anomer cyclized pyranose Haworth projection of the below hexose. Circle the anomeric carbons. Number the carbons on the Fischer and Haworth projections. Assign R and S for each chiral center. HO CHO -H HO -H H- -OH H -OH CH₂OH Draw the ẞ-anomer cyclized furanose Haworth projection for the below hexose. Circle the anomeric carbons. Number the carbons on the Fischer and Haworth projections. HO CHO -H H -OH HO -H H -OH CH₂OHarrow_forwardName the below disaccharide. Circle any hemiacetals. Identify the numbering of glycosidic linkage, and identify it as a or ẞ. OH HO HO OH HO HO HO OHarrow_forward
- What are the monomers used to make the following polymers? F. а. b. с. d. Вецер хочому なarrow_forward1. Propose a reasonable mechanism for the following transformation. I'm looking for curved mechanistic arrows and appropriate formal charges on intermediates. OMe MeO OMe Me2N NMe2 OTBS OH xylenes OMe 'OTBSarrow_forwardWhat is the polymer made from the following monomers? What type of polymerization is used for each? а. ОН H2N но b. ن -NH2 d. H₂N NH2 довarrow_forward
- Condensation polymers are produced when monomers containing two different functional groups link together with the loss of a small molecule such as H2O. The difunctional monomer H2N(CH2)6COOH forms a condensation polymer. Draw the carbon-skeleton structure of the dimer that forms from this monomer.arrow_forwardWhat is the structure of the monomer?arrow_forward→ BINDERIYA GANBO... BINDERIYA GANBO. AP Biology Notes Gamino acid chart - G... 36:22 司 10 ☐ Mark for Review Q 1 Hide 80 8 2 =HA O=A¯ = H₂O Acid HIO HBrO HCIO Question 10 of 35 ^ Σ DELL □ 3 % Λ & 6 7 * ∞ 8 do 5 $ 4 # m 3 ° ( 9 Highlights & Notes AXC Sign out Carrow_forward
- Which representation(s) show polymer structures that are likely to result in rigid, hard materials and those that are likely to result in flexible, stretchable, soft materials?arrow_forward3. Enter the molecular weight of the product obtained from the Williamson Ether Synthesis? OH OH & OH excess CH3l Ag₂Oarrow_forwardPlease answer 1, 2 and 3 on the endarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





