
Concept explainers
For each amino acid: [1] draw the L enantiomer in a Fischer projection; [2] classify the amino acid as neutral, acidic, or basic; [3) give the three-letter symbol; [4] give the one-letter symbol.
- arginine
- tyrosine
- glutamic acid
- valine
(a)
Interpretation:
The L-isomer of arginine in Fisher projection needs to be drawn and identified as acidic, basic or neutral. The three letter and one symbol for it needs to be determined.
Concept Introduction:
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral center of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
Answer to Problem 26P
- L isomer of arginine is as follows:
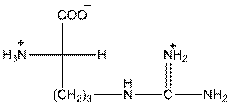
- In arginine, an additional basic nitrogen present. So, it is basic in nature.
- Three-letter symbol for arginine is 'Arg'.
- One letter symbol is 'R'.
Explanation of Solution
Amino acids that have the
To draw the Fisher projection of arginine, place the
at the bottom and to draw L isomer draw
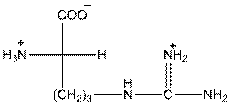
Here, R group is as follows:
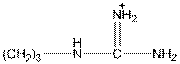
In arginine, an additional basic nitrogen present. So, it is basic in nature.
Three-letter symbol for arginine is Arg and one letter symbol is R.
(b)
Interpretation:
The L-isomer of tyrosine in Fisher projection needs to be drawn and identified as acidic, basic or neutral. The three-letter and one symbol for it needs to be determined.
Concept Introduction:
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral centre of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
Answer to Problem 26P
- L-isomer of tyrosine is as follows:
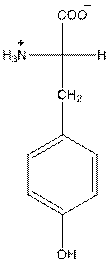
- Tyrosine is neutral in nature.
- Three-letter symbol for tyrosine is 'Tyr'.
- One letter symbol for tyrosine is 'Y'.
Explanation of Solution
Amino acids that have the
To draw the Fisher projection of tyrosine by placing the
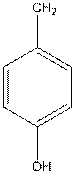
at the bottom and to draw L isomer draw
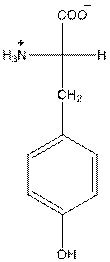
The overall charge of tyrosine is zero as there is one positive and negative charge. Hence, tyrosine is neutral in nature.
Three-letter symbol for tyrosine is 'Tyr' and one letter symbol is 'Y'.
(c)
Interpretation:
The L-isomer of glutamic acid in Fisher projection needs to be drawn and identified as acidic, basic or neutral. The three-letter and one symbol for it needs to be determined.
Concept Introduction:
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral centre of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
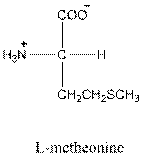
Answer to Problem 26P
- L-isomer of glutamic acid is as follows:
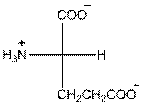
- Glutamic acid is acidic in nature.
- Three-letter symbol for glutamic acid is 'Glu'.
- One letter symbol for glutamic acid is 'E'.
Explanation of Solution
Amino acids that have the
Draw the Fisher projection of glutamic acid by placing the
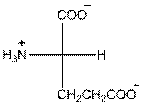
In glutamic acid, one additional acidic carboxyl group present. So, glutamic acid is acidic in nature.
Three-letter symbol for glutamic acid is 'Glu' and one letter symbol for glutamic acid is 'E'.
(d)
Interpretation:
The L-isomer of valine in Fisher projection needs to be drawn and identified as acidic, basic or neutral. The three-letter and one symbol for it needs to be determined.
Concept Introduction:
Like monosaccharides, the prefixes D and L are used to designate the arrangement of groups to the chiral centre of amino acid, when drawn with a vertical carbon chain having
Amino acids that have the
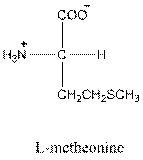
Answer to Problem 26P
- L-isomer of valine is as follows:
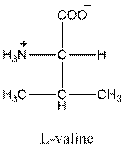
- Valine is neutral in nature.
- Three-letter symbol for valine is 'Val'.
- One letter symbol for valine is 'V'.
Explanation of Solution
Amino acids that have the
Draw the Fisher projection of valine by placing the
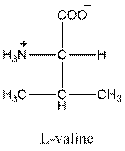
The net charge in valine is zero as there is one positive charge and negative charge in the compound. So, valine is neutral.
Three-letter symbol for valine is 'Val' and one letter symbol for valine is 'V'.
Want to see more full solutions like this?
Chapter 21 Solutions
Connect One Semester Access Card for General, Organic, & Biological Chemistry
- (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B Bond A Bond C a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. Weakest Bond Strongest Bond b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. c. (5pts) Use principles discussed in lecture, supported by relevant structures, to succinctly explain the why your part b (i) radical is more stable than your part b(ii) radical. Written explanation can be no more than one-two succinct sentence(s)!arrow_forward. 3°C with TH 12. (10pts total) Provide the major product for each reaction depicted below. If no reaction occurs write NR. Assume heat dissipation is carefully controlled in the fluorine reaction. 3H 24 total (30) 24 21 2h • 6H total ● 8H total 34 래 Br2 hv major product will be most Substituted 12 hv Br NR I too weak of a participate in P-1 F₂ hv Statistically most favored product will be major = most subst = thermo favored hydrogen atom abstractor to LL Farrow_forwardFive chemistry project topic that does not involve practicalarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardQ2. Consider the hydrogenation of ethylene C2H4 + H2 = C2H6 The heats of combustion and molar entropies for the three gases at 298 K are given by: C2H4 C2H6 H2 AH comb/kJ mol¹ -1395 -1550 -243 Sº / J K¹ mol-1 220.7 230.4 131.1 The average heat capacity change, ACP, for the reaction over the temperature range 298-1000 K is 10.9 J K¹ mol¹. Using these data, determine: (a) the standard enthalpy change at 800 K (b) the standard entropy change at 800 K (c) the equilibrium constant at 800 K.arrow_forward13. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B Bond A Bond C a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. Weakest Bond Strongest Bond b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. c. (5pts) Use principles discussed in lecture, supported by relevant structures, to succinctly explain the why your part b (i) radical is more stable than your part b(ii) radical. Written explanation can be no more than one-two succinct sentence(s)! Googlearrow_forward
- Print Last Name, First Name Initial Statifically more chances to abstract one of these 6H 11. (10pts total) Consider the radical chlorination of 1,3-diethylcyclohexane depicted below. 4 4th total • 6H total 래 • 4H total 21 total ZH 2H Statistical H < 3° C-H weakest - product abstraction here bund leads to thermo favored a) (6pts) How many unique mono-chlorinated products can be formed and what are the structures for the thermodynamically and statistically favored products? Product 6 Number of Unique Mono-Chlorinated Products Thermodynamically Favored Product Statistically Favored Product b) (4pts) Draw the arrow pushing mechanism for the FIRST propagation step (p-1) for the formation of the thermodynamically favored product. Only draw the p-1 step. You do not need to include lone pairs of electrons. No enthalpy calculation necessary H H-Cl Waterfoxarrow_forward10. (5pts) Provide the complete arrow pushing mechanism for the chemical transformation → depicted below Use proper curved arrow notation that explicitly illustrates all bonds being broken, and all bonds formed in the transformation. Also, be sure to include all lone pairs and formal charges on all atoms involved in the flow of electrons. CH3O II HA H CH3O-H H ①arrow_forwardDo the Lone Pairs get added bc its valence e's are a total of 6 for oxygen and that completes it or due to other reasons. How do we know the particular indication of such.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





