
Pearson eText Organic Chemistry -- Instant Access (Pearson+)
9th Edition
ISBN: 9780135213728
Author: Leroy Wade, Jan Simek
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 21.49SP
Propose mechanisms for the following reactions.
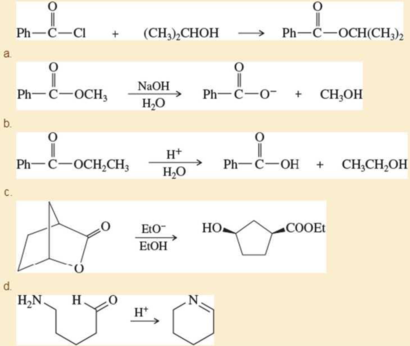
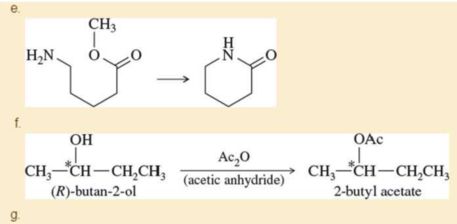
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Complete the equation...see image
Complete the equation...see image
Complete the equation...see photo
Chapter 21 Solutions
Pearson eText Organic Chemistry -- Instant Access (Pearson+)
Ch. 21.2F - Name the following carboxylic acid derivatives,...Ch. 21.4A - Prob. 21.2PCh. 21.4A - Prob. 21.3PCh. 21.4A - Prob. 21.4PCh. 21.5C - Prob. 21.7PCh. 21.6 - When ethyl 4-hydroxybutyrate is heated in the...Ch. 21.6 - Propose a mechanism for the following ring-opening...Ch. 21.6 - Prob. 21.15PCh. 21.7B - Prob. 21.16PCh. 21.7C - Prob. 21.19P
Ch. 21.7C - Prob. 21.20PCh. 21.7C - Prob. 21.21PCh. 21.7D - Prob. 21.22PCh. 21.7D - The mechanism for acidic hydrolysis of a nitrile...Ch. 21.8A - Prob. 21.24PCh. 21.8C - Prob. 21.25PCh. 21.9 - Prob. 21.26PCh. 21.9 - Prob. 21.27PCh. 21.9 - Prob. 21.28PCh. 21.10 - Draw a mechanism for the acylation of anisole by...Ch. 21.10 - Prob. 21.30PCh. 21.11 - Prob. 21.31PCh. 21.11 - Prob. 21.32PCh. 21.12 - Problem 21-33 Propose a mechanism for the...Ch. 21.12 - Suggest the most appropriate reagent for each...Ch. 21.12 - Show how you would synthesize each compound,...Ch. 21.13 - Prob. 21.36PCh. 21.13 - Prob. 21.37PCh. 21.14 - Prob. 21.38PCh. 21.14 - Prob. 21.39PCh. 21.16 - Prob. 21.40PCh. 21.16 - Prob. 21.41PCh. 21 - Prob. 21.42SPCh. 21 - Give appropriate names for the following...Ch. 21 - Predict the major products formed when benzoyl...Ch. 21 - Predict the products of the following reactions....Ch. 21 - Prob. 21.46SPCh. 21 - Prob. 21.47SPCh. 21 - Prob. 21.48SPCh. 21 - Propose mechanisms for the following reactions.Ch. 21 - Prob. 21.51SPCh. 21 - An ether extraction of nutmeg gives large...Ch. 21 - Prob. 21.53SPCh. 21 - Show how you would accomplish the following...Ch. 21 - Prob. 21.55SPCh. 21 - Prob. 21.56SPCh. 21 - Prob. 21.57SPCh. 21 - Prob. 21.58SPCh. 21 - Prob. 21.59SPCh. 21 - Explain this curious result. What does this...Ch. 21 - Prob. 21.61SPCh. 21 - Prob. 21.62SPCh. 21 - Prob. 21.63SPCh. 21 - A chemist was called to an abandoned aspirin...Ch. 21 - Prob. 21.67SPCh. 21 - The IR spectrum, 13ONTVTR spectrum, and 1HNMR...Ch. 21 - Prob. 21.69SPCh. 21 - Prob. 21.70SPCh. 21 - Prob. 21.71SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please see photoarrow_forward=Naming benzene derivatives Name these organic compounds: structure C1 CH3 name ☐ CH3 ப C1 × ☐arrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see image **NOTE: The compound on the left is the starting point, and the compound on the right is the final product. Please show the steps in between to get from start to final, please. These are not two different compounds that need to be worked.arrow_forward
- Nucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor” *see attachedarrow_forwardNucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forward
- Show the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forwardDraw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License