
Concept explainers
(a)
Interpretation: The number of stereogenic centers present in
Concept introduction: Carbohydrates are naturally occurring compounds. Carbohydrates are polyhydroxy
Answer to Problem 21.39P
There are five stereogenic centers present in
Explanation of Solution
The stereogenic centers in
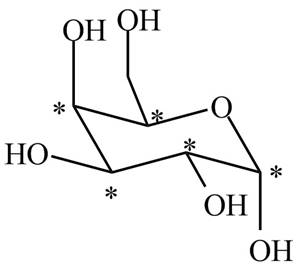
Figure 1
The stereogenic centers are marked by star. There are five stereogenic centers present in
There are five stereogenic centers present in
(b)
Interpretation: The hemiacetal carbon in
Concept introduction: Carbohydrates are naturally occurring compounds. Carbohydrates are polyhydroxy aldehydes and ketones. Galactose is a aldohexose as it contains six carbon atoms as well as an aldehyde functional group. The molecular formula of galactose
Aldehydes or ketones on reaction with one equivalent of alcohol form hemiacetal and on reaction with two equivalents of alcohol it forms acetals. This is nucleophilic addition reaction. These reactions takes place in presence of acids, commonly
Ethers contain only one alkoxy group on a carbon atom while acetals contain two alkoxy groups on a single carbon atom.
Hemiacetals contains one alkoxy group and one hydroxyl group attached to same carbon atom.
Answer to Problem 21.39P
The hemiacetal carbon in
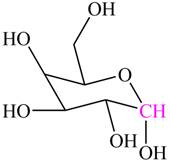
Explanation of Solution
The hemiacetal carbon in
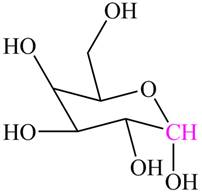
Figure 2
The highlighted carbon contains alkoxy group and hydroxyl group. Hence, this carbon is labeled as hemiacetal carbon.
The hemiacetal carbon in
(c)
Interpretation: The structure of
Concept introduction: Carbohydrates are naturally occurring compounds. Carbohydrates are polyhydroxy aldehydes and ketones. Galactose is a aldohexose as it contains six carbon atoms as well as an aldehyde functional group. The molecular formula of galactose
Answer to Problem 21.39P
The structure of
Explanation of Solution
In
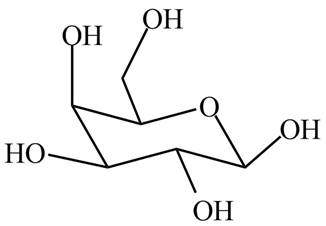
Figure 3
The structure of
(d)
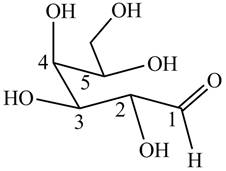
Interpretation: The structure of poly hydroxy aldehyde that cyclizes to
Concept introduction: Carbohydrates are naturally occurring compounds. Carbohydrates are polyhydroxy aldehydes and ketones. Galactose is a aldohexose as it contains six carbon atoms as well as an aldehyde functional group. The molecular formula of galactose
Aldehydes or ketones on reaction with one equivalent of alcohol form hemiacetal and on reaction with two equivalents of alcohol it forms acetals. This is nucleophilic addition reaction. These reactions takes place in presence of acids, commonly
In
Answer to Problem 21.39P
The structure of poly hydroxy aldehyde that cyclizes to
Explanation of Solution
The cyclization of poly hydroxy aldehyde results in the formation of hemiacetal. The hydroxyl group on

Figure 4
The structure of poly hydroxy aldehyde that cyclizes to
(e)
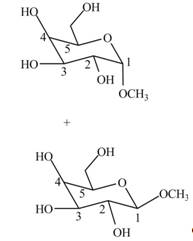
Interpretation: The products formed when
Concept introduction: Carbohydrates are naturally occurring compounds. Carbohydrates are polyhydroxy aldehydes and ketones. Galactose is a aldohexose as it contains six carbon atoms as well as an aldehyde functional group. The molecular formula of galactose
Aldehydes or ketones on reaction with one equivalent of alcohol form hemiacetal and on reaction with two equivalents of alcohol it forms acetals. This is nucleophilic addition reaction. These reactions takes place in presence of acids, commonly
Answer to Problem 21.39P
The products formed when
Explanation of Solution
Cyclic hemiacetals can be converted to acetals by treatment with alcohol in presence of acid. The hydroxyl group of hemiacetal is converted to alkoxy group. The
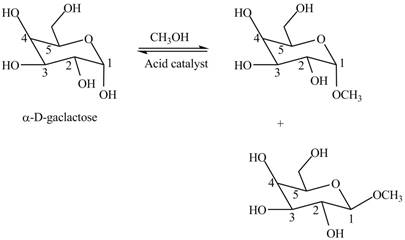
Figure 5
The products formed when
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry
- Name the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forwardMacmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forward
- Provide the reagents for the following reactions.arrow_forwardIf I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





