
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 15P
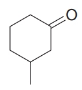
How would you synthesize each of the following using a Gilman reagent?
(a)
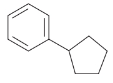
(b)
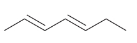
(c)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Predict the major products of this reaction:
excess
H+
NaOH
?
A
Note that the first reactant is used in excess, that is, there is much more of the first reactant than the second.
If there won't be any products, just check the box under the drawing area instead.
Explanation
Check
Click and drag to start drawing a
structure.
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Priv
1. For each of the reaction "railroads" below, you are either asked to give the structure(s) of the starting
material(s) or product(s), or provide reagents/conditions to accomplish the transformation, as
indicated by the boxes.
a.
NaOMe
H+
.CO,H
HO₂C
MeOH (excess)
MeOH
H3C
Br
يع
CH3
1. LiAlH4
2. H3O+
3. PBг3
H3C
1. Et-Li
2. H3O+
-CO₂Me
-CO₂Me
OH
CH3
CH3
ল
CH3
Predict the intermediate 1 and final product 2 of this organic reaction:
NaOMe
ག1, ད།་, -
+
H
You can draw 1 and 2 in any arrangement you like.
2
work up
Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge)
bonds at the chiral center.
Explanation
Check
Click and drag to start drawing a structure.
Х
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | P
Chapter 21 Solutions
Organic Chemistry
Ch. 21 - PRACTICE PROBLEM 21.1
For each of the following...Ch. 21 - Prob. 2PPCh. 21 - PRACTICE PROBLEM 21.3 What product would you...Ch. 21 - Prob. 4PPCh. 21 - PRACTICE PROBLEM 21.5 What is the product of the...Ch. 21 - Prob. 6PPCh. 21 - Prob. 7PPCh. 21 - Prob. 8PPCh. 21 - Prob. 9PPCh. 21 - Prob. 10PP
Ch. 21 - Prob. 11PPCh. 21 - Practice Problem 21.12 What products would form...Ch. 21 - Prob. 13PCh. 21 - Prob. 14PCh. 21 - How would you synthesize each of the following...Ch. 21 - Prob. 16PCh. 21 - Predict the product(s) for each of the following...Ch. 21 - Prob. 18PCh. 21 - Prob. 19PCh. 21 - Prob. 20PCh. 21 - Prob. 21PCh. 21 - 21.22 Write a mechanism that can account for the...Ch. 21 - Prob. 23PCh. 21 - Prob. 24PCh. 21 - Prob. 25PCh. 21 - 21.26 In 1985, T. Katz (Columbia University)...Ch. 21 - When the following molecule was exposed to the...Ch. 21 - During the course of the following Stille...Ch. 21 - 1. In “The Chemistry of... Complex Cross...Ch. 21 - Prob. 2LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Which fibrous joints are synarthroses? Which are amphiarthroses?
Principles of Anatomy and Physiology
An object, with mass m and speed v relative to an observer, explodes into two pieces, one three times as massiv...
Fundamentals of Physics Extended
Use the following graph to answer questions 3 and 4. 3. Which of the lines best depicts the log phase of a ther...
Microbiology: An Introduction
The symbol of an ion with 22 protons, 24 neutrons and 18 electrons needs to be determined. Concept introduction...
Living By Chemistry: First Edition Textbook
8. A human maintaining a vegan diet (containing no animal products) would be a:
a. producer
b. primary consume...
Human Biology: Concepts and Current Issues (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the total energy cost associated with the compound below adopting the shown conformation? CH3 HH DH CH3arrow_forwardΗΝ, Draw Final Product C cyclohexanone pH 4-5 Edit Enamine H3O+ CH3CH2Br THF, reflux H Edit Iminium Ionarrow_forwardHow many hydrogen atoms are connected to the indicated carbon atom?arrow_forward
- Identify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forward
- Rank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forward
- Why isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY