
Concept explainers
a)
Interpretation:
How to carry out the transformation given, benzene to 2-methyl-2-phenylpropanoic acid, is to be shown.
Concept introduction:
The conversion of benzene to 2-methyl-2-phenylpropanoicacid involves the following steps. i) Conversion of benzene to isopropylbenzene by Friedal-Crafts alkylation. ii) Introduction of bromine in the benzyl carbon using NBS. iii) Conversion to a Grignard reagent iv) Treatment with CO2 followed by hydrolysis.
To show:
How to transform benzene to 2-methyl-2-phenylpropanoic acid.
b)
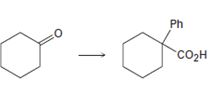
Interpretation:
How to carry out the transformation given, conversion of cyclohexanone to 1-phenylcyclohexanecarboxylic acid, is to be shown.
Concept introduction:
The steps involved in the conversion of cyclohexanone to 1-phenylcyclohexanecarboxylic acid are i) conversion of the
To show:
How to carry out the conversion of cyclohexanone to 1-phenylcyclohexanecarboxylic acid.
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
- Indicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol.arrow_forward2,2-Dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol. Indicate the products obtained.arrow_forward
- Add conditions above and below the arrow that turn the reactant below into the product below in a single transformationADS fint anditions 百 Abl res condinese NC ง Add on condtions 1.0 B H,N.arrow_forward3. Provide all the steps and reagents for this synthesis. OHarrow_forwardSteps and explanationarrow_forward
- Steps and explanations please.arrow_forwardSteps on how to solve. Thank you!arrow_forward3. Name this ether correctly. H₁C H3C CH3 CH3 4. Show the best way to make the ether in #3 by a Williamson Ether Synthesis. Start from an alcohol or phenol. 5. Draw the structure of an example of a sulfide.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

