
Concept explainers
(a)
Interpretation:
Formula for the octahedral complex ion has to be written that has
(a)
Explanation of Solution
Octahedral complex contains six ligands inside the coordination sphere. Central metal atom is given as
(b)
Interpretation:
Complex that is formed from
(b)
Explanation of Solution
Octahedral complex ion that is formed from
Octahedral complex ion that is formed from
(c)
Interpretation:
Complex that has more paramagnetism has to be chosen.
(c)
Explanation of Solution
Octahedral complex ion that is formed from
Octahedral complex ion that is formed from
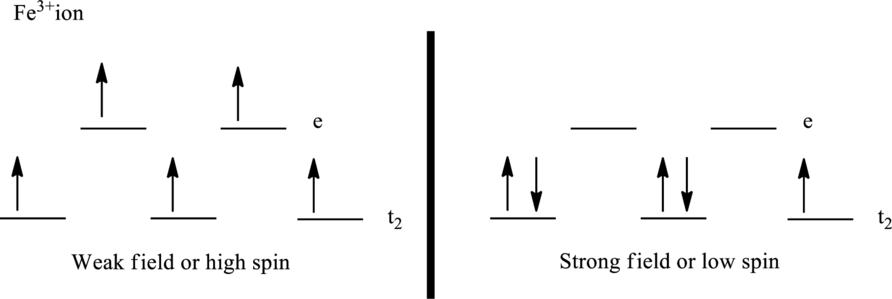
The crystal field model for high-spin and low-spin complex of
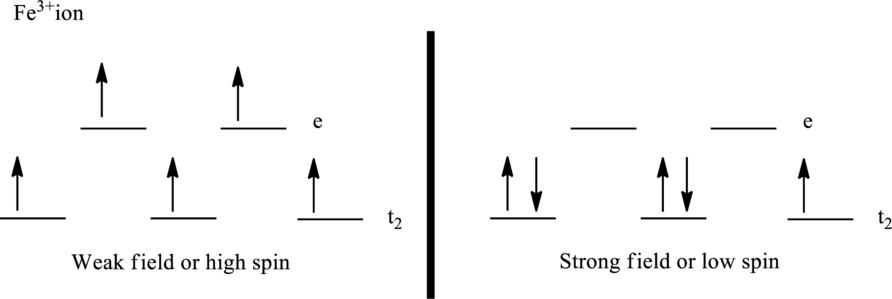
Number of unpaired electrons:
The ligand
Paramagnetism depends upon the number of unpaired electrons. Therefore, the complex ion
(c)
Interpretation:
Crystal field diagram for the
(c)
Explanation of Solution
Octahedral complex ion that is formed from
Octahedral complex ion that is formed from
The central metal ion is
Want to see more full solutions like this?
Chapter 20 Solutions
Chemistry: The Molecular Science
- NAME: 1. Draw the major product of the following E2 reaction. Make sure you pay attention to REGIOCHEMISTRY and STEREOCHEMISTRY. To get credit for this question, you must EXPLAIN how you got your answer using STRUCTURES and WORDS. Br NaOCH3 acetone F2 reaction To get credit for thisarrow_forward3. Reactions! Fill in the information missing below. Make sure to pay attention to REGIOCHEMISTRY and STEREOCHEMISTRY. Br2 CH3OH + 4. Mechanism! Show the complete arrow pushing mechanism, including all steps and intermediates for the following reactions. To get credit for this, you MUST show how ALL bonds are broken and formed, using arrows to show the movement of electrons. H3O+ HOarrow_forwardPlease provide a synthesis for the Ester using proponoic acid, thank you!arrow_forward
- Please help with the curved arrow mechanism of this reaction, thank youarrow_forwardConcentration (mg/l) Peak Area 0 158 10 10241 20 18425 30 26457 40 37125 50 44256 60 56124 Question: Determine the regression equation (a and b coefficients) from first principlesarrow_forwardConcentration (mg/l) Peak Area 0 158 10 10241 20 18425 30 26457 40 37125 50 44256 60 56124 You have been asked to determine the concentration of citral in a highly valued magnolia essential oil. QUESTION: Calculate the concentration of citral in your highly valued magnolia essential oil which returns a peak area of 41658arrow_forward
- Need help with these problems...if you can please help me understand problems E & F.arrow_forwardPlease help me solve these problems. Thank you in advance.arrow_forwardPredict the products of this organic reaction: O N IN A N + H2O + HCI ? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. 田 C + Explanation Check Click and drag to start drawing a structure. C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- 6. For each of the following, fill in the synthesis arrows with reagents and show the intermediates. You DO NOT need to use the same number of arrows that are shown (you may use more or less), but the product must be formed from the reactant. Then write the mechanism of one step in the synthesis (you can choose which step to write the mechanism for), including all reagents required, clearly labeling the nucleophile and electrophile for each step, and using curved arrows to show the steps in the mechanism. a. b. OHarrow_forwardDraw the productsarrow_forwardDraw the correct productsarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





