
Pearson eText -- Physics for Scientists and Engineers with Modern Physics -- Instant Access (Pearson+)
5th Edition
ISBN: 9780137488179
Author: Douglas Giancoli
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 20, Problem 48P
(II) An ideal gas of n moles undergoes the reversible process ab shown in the PV diagram of Fig. 20–20. The temperature Τ of the gas is the same at points a and b. Determine the change in entropy of the gas due to this process.
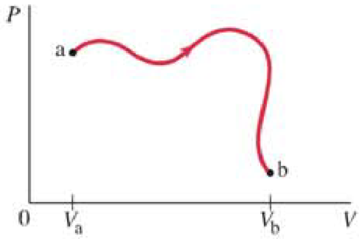
FIGURE 20–20
Problem 44.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The kinetic energy of a pendulum is greatest
Question 20Select one:
a.
at the top of its swing.
b.
when its potential energy is greatest.
c.
at the bottom of its swing.
d.
when its total energy is greatest.
Part a-D pl
The figure (Figure 1) shows representations of six
thermodynamic states of the same ideal gas sample.
Figure
1 of 1
Part A
■Review | Constants
Rank the states on the basis of the pressure of the gas sample at each state.
Rank pressure from highest to lowest. To rank items as equivalent, overlap them.
▸ View Available Hint(s)
highest
0
☐ ☐ ☐ ☐ ☐ ☐
Reset
Help
B
F
A
D
E
The correct ranking cannot be determined.
Submit
Previous Answers
× Incorrect; Try Again; 4 attempts remaining
Provide Feedback
lowest
Next >
Chapter 20 Solutions
Pearson eText -- Physics for Scientists and Engineers with Modern Physics -- Instant Access (Pearson+)
Ch. 20.3 - Prob. 1AECh. 20.9 - Prob. 1DECh. 20 - Prob. 1QCh. 20 - Can you warm a kitchen in winter by leaving the...Ch. 20 - Would a definition of heat engine efficiency as e...Ch. 20 - Prob. 4QCh. 20 - Prob. 5QCh. 20 - The oceans contain a tremendous amount of thermal...Ch. 20 - Discuss the factors that keep real engines from...Ch. 20 - Prob. 8Q
Ch. 20 - Describe a process in nature that is nearly...Ch. 20 - (a) What happens if you remove the lid of a bottle...Ch. 20 - Prob. 11QCh. 20 - Prob. 12QCh. 20 - Give three examples, other than those mentioned in...Ch. 20 - Which do you think has the greater entropy, 1 kg...Ch. 20 - Prob. 16QCh. 20 - Prob. 17QCh. 20 - The first law of thermodynamics is sometimes...Ch. 20 - Powdered milk is very slowly (quasistatically)...Ch. 20 - Two identical systems are taken from state a to...Ch. 20 - It can he said that the total change in entropy...Ch. 20 - Prob. 22QCh. 20 - Prob. 23QCh. 20 - Prob. 1MCQCh. 20 - Prob. 2MCQCh. 20 - Prob. 3MCQCh. 20 - Prob. 4MCQCh. 20 - Prob. 5MCQCh. 20 - Prob. 6MCQCh. 20 - Prob. 7MCQCh. 20 - Prob. 8MCQCh. 20 - Prob. 9MCQCh. 20 - Prob. 10MCQCh. 20 - Prob. 11MCQCh. 20 - Prob. 12MCQCh. 20 - Prob. 1PCh. 20 - Prob. 2PCh. 20 - Prob. 3PCh. 20 - (II) A typical compact car experiences a total...Ch. 20 - Prob. 5PCh. 20 - (II) Figure 2017 is a PV diagram for a reversible...Ch. 20 - Prob. 7PCh. 20 - Prob. 8PCh. 20 - Prob. 9PCh. 20 - Prob. 10PCh. 20 - (II) (a) Show that the work done by a Carnot...Ch. 20 - Prob. 12PCh. 20 - Prob. 13PCh. 20 - Prob. 14PCh. 20 - (II) Assume that a 65 kg hiker needs 4.0 103 kcal...Ch. 20 - Prob. 16PCh. 20 - Prob. 18PCh. 20 - (III) A Carnot cycle, shown in Fig. 20-7, has the...Ch. 20 - (III) One mole of monatomic gas undergoes a Carnot...Ch. 20 - (III) In an engine that approximates the Otto...Ch. 20 - Prob. 22PCh. 20 - Prob. 23PCh. 20 - Prob. 24PCh. 20 - Prob. 25PCh. 20 - Prob. 26PCh. 20 - Prob. 27PCh. 20 - Prob. 28PCh. 20 - (II) An ideal heal pump is used to maintain the...Ch. 20 - Prob. 30PCh. 20 - Prob. 31PCh. 20 - Prob. 32PCh. 20 - Prob. 33PCh. 20 - Prob. 34PCh. 20 - Prob. 35PCh. 20 - (I) What is the change in entropy of 1.00 m3 of...Ch. 20 - Prob. 37PCh. 20 - (II) If 0.45kg f water at 100C is changed by a...Ch. 20 - Prob. 39PCh. 20 - Prob. 40PCh. 20 - Prob. 41PCh. 20 - Prob. 42PCh. 20 - Prob. 43PCh. 20 - Prob. 44PCh. 20 - Prob. 45PCh. 20 - Prob. 46PCh. 20 - Prob. 47PCh. 20 - (II) An ideal gas of n moles undergoes the...Ch. 20 - Prob. 49PCh. 20 - Prob. 50PCh. 20 - (II) Two samples of an ideal gas are initially at...Ch. 20 - (II) 1.00 mole of nitrogen (N2) gas and 1.00 mole...Ch. 20 - (II) (a) Why would you expect the total entropy...Ch. 20 - (II) Thermodynamic processes are sometimes...Ch. 20 - Prob. 55PCh. 20 - (III) Consider an ideal gas of n moles with molar...Ch. 20 - (III) A general theorem states that the amount of...Ch. 20 - Prob. 58PCh. 20 - (I) Use Eq. 2014 to determine the entropy of each...Ch. 20 - (II) Suppose that you repeatedly shake six coins...Ch. 20 - (II) (a) Suppose you have four coins, all with...Ch. 20 - Prob. 62PCh. 20 - Prob. 63PCh. 20 - Prob. 64PCh. 20 - Prob. 65PCh. 20 - Prob. 66PCh. 20 - Prob. 67GPCh. 20 - Prob. 68GPCh. 20 - A heat engine takes a diatomic gas around the...Ch. 20 - Prob. 70GPCh. 20 - Prob. 71GPCh. 20 - Prob. 72GPCh. 20 - The operation of a certain heat engine takes an...Ch. 20 - Prob. 74GPCh. 20 - Prob. 75GPCh. 20 - 1.00 mole of an ideal monatomic gas at STP first...Ch. 20 - Prob. 77GPCh. 20 - Prob. 78GPCh. 20 - Prob. 80GPCh. 20 - Prob. 82GPCh. 20 - The Stirling cycle shown in Fig 20-27, is useful...Ch. 20 - Prob. 84GPCh. 20 - Prob. 85GPCh. 20 - Thermodynamic processes can be represented not...Ch. 20 - An aluminum can, with negligible heat capacity, is...Ch. 20 - Prob. 88GPCh. 20 - A bowl contains a large number of red, orange, and...Ch. 20 - Prob. 90GPCh. 20 - Prob. 92GP
Additional Science Textbook Solutions
Find more solutions based on key concepts
3. CAUTION Why is genetic drift aptly named?
a. It causes allele frequencies to drift up or down randomly.
b. I...
Biological Science (6th Edition)
60. You are 9.0 m from the door of your bus, behind the bus, when it pulls away with an acceleration of 1.0 m/...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
All of the following processes are involved in the carbon cycle except: a. photosynthesis b. cell respiration c...
Human Biology: Concepts and Current Issues (8th Edition)
Distinguish between microevolution, speciation, and macroevolution.
Campbell Essential Biology (7th Edition)
If someone at the other end of a room smokes a cigarette, you may breathe in some smoke. The movement of smoke ...
Campbell Essential Biology with Physiology (5th Edition)
10. Suppose you are holding a box in front of you and away from your body by squeezing the sides, as shown in F...
College Physics: A Strategic Approach (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Part A m 2πkT ) 3/2 Calculate the integral (v) = f vƒ (v)dv. The function f(v) describing the actual distribution of molecular speeds is called the Maxwell-Boltzmann distribution, = ƒ(v) = 4π (· v²e-mv²/2kT . (Hint: Make the change of variable v² =x and use the tabulated integral foxne integer and a is a positive constant.) Express your answer in terms of the variables T, m, and appropriate constants. -ax dx n! - an+1 where n is a positive (v) = ΕΠΙ ΑΣΦ Submit Previous Answers Request Answer ? × Incorrect; Try Again; 4 attempts remaining Al Study Tools Looking for some guidance? Let's work through a few related practice questions before you go back to the real thing. This won't impact your score, so stop at anytime and ask for clarification whenever you need it. Ready to give it a try? Startarrow_forwardStarter the rule of significantarrow_forwardPlease solve this problem and give step by step explanations on each step while breaking it down please. Thank you!!arrow_forward
- Car A starts from rest at t = 0 and travels along a straight road with a constant acceleration of 6 ft/s^2 until it reaches a speed of 60ft/s. Afterwards it maintains the speed. Also, when t = 0, car B located 6000 ft down the road is traveling towards A at a constant speed of 80 ft/s. Determine the distance traveled by Car A when they pass each other.Write the solution using pen and draw the graph if needed.arrow_forwardIn the given circuit the charge on the plates of 1 μF capacitor, when 100 V battery is connected to the terminals A and B, will be 2 μF A 1 µF B 3 µFarrow_forwardThe velocity of a particle moves along the x-axis and is given by the equation ds/dt = 40 - 3t^2 m/s. Calculate the acceleration at time t=2 s and t=4 s. Calculate also the total displacement at the given interval. Assume at t=0 s=5m.Write the solution using pen and draw the graph if needed.arrow_forward
- The velocity of a particle moves along the x-axis and is given by the equation ds/dt = 40 - 3t^2 m/s. Calculate the acceleration at time t=2 s and t=4 s. Calculate also the total displacement at the given interval. Assume at t=0 s=5m.Write the solution using pen and draw the graph if needed.arrow_forwardThe velocity of a particle moves along the x-axis and is given by the equation ds/dt = 40 - 3t^2 m/s. Calculate the acceleration at time t=2 s and t=4 s. Calculate also the total displacement at the given interval. Assume at t=0 s=5m.Write the solution using pen and draw the graph if needed. NOT AI PLSarrow_forwardThe velocity of a particle moves along the x-axis and is given by the equation ds/dt = 40 - 3t^2 m/s. Calculate the acceleration at time t=2 s and t=4 s. Calculate also the total displacement at the given interval. Assume at t=0 s=5m.Write the solution using pen and draw the graph if needed.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY