
(a)
Interpretation: The
Concept introduction:
Ocean acidification: The increase in concentration of carbon-dioxide in the atmosphere leads to ocean acidification. The amount is increasing day by day and this increase will lead to higher concentrations of dissolved
The concentration of hydronium ion is a key factor for many biochemical reactions. So variation may affect the organisms in the oceans.
The relationship between ocean
(a)
Answer to Problem 40GQ
The
Explanation of Solution
If the concentration of dissolved
The graph showing the fraction of species in the solution as a function of
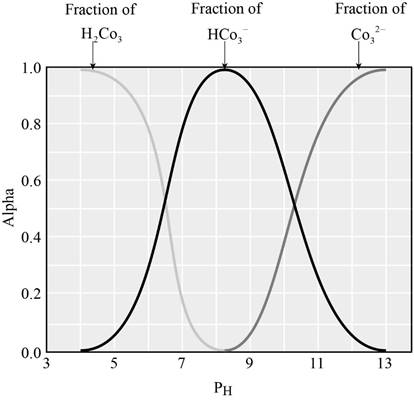
From the graph we can identify the
The fraction of dissociation will be 0.50 when the concentration of both the species will be equal.
The
(b)
Interpretation: The
Concept introduction:
Ocean acidification: The increase in concentration of carbon-dioxide in the atmosphere leads to ocean acidification. The amount is increasing day by day and this increase will lead to higher concentrations of dissolved
The concentration of hydronium ion is a key factor for many biochemical reactions. So variation may affect the organisms in the oceans.
The relationship between ocean
(b)
Answer to Problem 40GQ
The
Explanation of Solution
If the concentration of dissolved
The graph showing the fraction of species in the solution as a function of
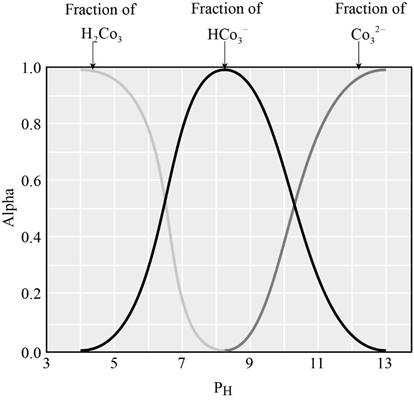
From the graph we can identify the
The fraction of dissociation will be 0.50 when the concentration of both the species will be equal.
The
(c)
Interpretation: The predominant species in the solution when the
Concept introduction:
Ocean acidification: The increase in concentration of carbon-dioxide in the atmosphere leads to ocean acidification. The amount is increasing day by day and this increase will lead to higher concentrations of dissolved
The concentration of hydronium ion is a key factor for many biochemical reactions. So variation may affect the organisms in the oceans.
The relationship between ocean
(c)
Answer to Problem 40GQ
The predominant species in the solution when the
Explanation of Solution
If the concentration of dissolved
The graph showing the fraction of species in the solution as a function of
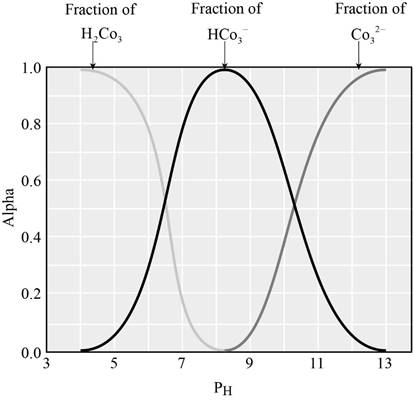
When the
From the graph it is clear that, the predominant species when the
(d)
Interpretation: The predominant species in the solution when the
Concept introduction:
Ocean acidification: The increase in concentration of carbon-dioxide in the atmosphere leads to ocean acidification. The amount is increasing day by day and this increase will lead to higher concentrations of dissolved
The concentration of hydronium ion is a key factor for many biochemical reactions. So variation may affect the organisms in the oceans.
The relationship between ocean
(d)
Answer to Problem 40GQ
The predominant species in the solution when the
Explanation of Solution
If the concentration of dissolved
The graph showing the fraction of species in the solution as a function of
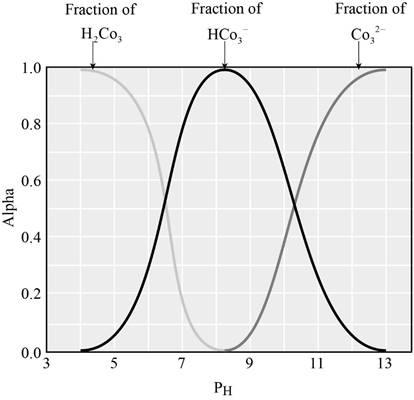
When the
From the graph it is clear that, the predominant species when the
Want to see more full solutions like this?
Chapter 20 Solutions
Chemistry & Chemical Reactivity
- Provide the reagents for the following reactions.arrow_forwardIf I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forward
- Indicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. C6H5 CH3arrow_forwardIf I have 1-bromopropene and I want to obtain (1,1-dipropoxyethyl)benzene, indicate the compound that I should add in addition to NaOH.arrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts. Ο HSCH2CH2CH2SH, BF3 Select to Draw I Submitarrow_forwardFeedback (7/10) Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. Incorrect, 3 attempts remaining Ο (CH3CH2)2NH, TSOH Select to Draw V N. 87% Retryarrow_forwardIf I want to obtain (1,1-dipropoxyethyl)benzene from 1-bromopropene, indicate the product that I have to add in addition to NaOH.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

