
Chemistry & Chemical Reactivity
9th Edition
ISBN: 9781133949640
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 29PS
In methane hydrate the methane molecule is trapped in a cage of water molecules. Describe the structure: (a) how many water molecules make up the cage, (b) how many hydrogen bonds are involved, and (c) how many faces does the cage have? (Figure 20 16.)
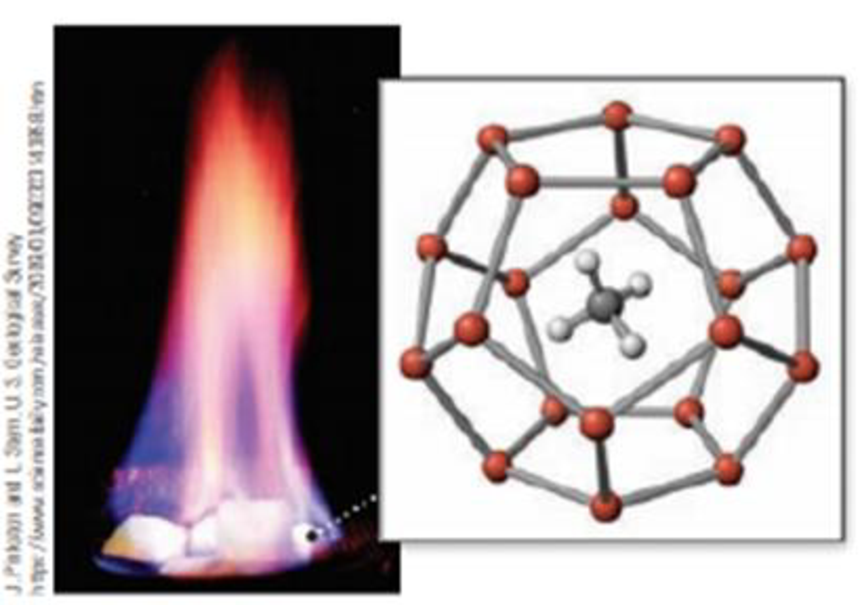
(a) Methane hydrate burns as methane gas escapes from the solid hydrate.
(b) Methane hydrate consists of a lattice of water molecules with methane molecules trapped in the cavity.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
help w d!
Help w b!
b.
CH3
H3C
'N'
H3C
CH3
CN
Ph
1.
OH
N
2. H2O2, Pyridine
Chapter 20 Solutions
Chemistry & Chemical Reactivity
Ch. 20.1 - Prob. 1RCCh. 20.1 - Prob. 2RCCh. 20.2 - Prob. 1RCCh. 20.2 - Prob. 2RCCh. 20.3 - Prob. 1RCCh. 20.3 - Prob. 2RCCh. 20.3 - Prob. 3RCCh. 20.4 - Prob. 1RCCh. 20.4 - Prob. 2RCCh. 20.4 - 3. Which of the following is a renewable energy...
Ch. 20.5 - Prob. 1RCCh. 20.5 - Prob. 2RCCh. 20.6 - Prob. 1QCh. 20.6 - Prob. 1RCCh. 20.7 - Prob. 1QCh. 20.7 - Prob. 2QCh. 20 - In the discussion on the composition of air,...Ch. 20 - Prob. 2PSCh. 20 - Prob. 3PSCh. 20 - Dinitrogen monoxide, N2O (commonly called nitrous...Ch. 20 - Prob. 5PSCh. 20 - Prob. 6PSCh. 20 - Prob. 9PSCh. 20 - Although there are a number of...Ch. 20 - Prob. 12PSCh. 20 - Prob. 13PSCh. 20 - Prob. 14PSCh. 20 - Prob. 15PSCh. 20 - Prob. 17PSCh. 20 - The enthalpy of combustion of isooctane (C8H18),...Ch. 20 - Energy consumption in the United States amounts to...Ch. 20 - Prob. 20PSCh. 20 - Prob. 23PSCh. 20 - Prob. 25PSCh. 20 - Prob. 28PSCh. 20 - In methane hydrate the methane molecule is trapped...Ch. 20 - Prob. 30PSCh. 20 - Prob. 31PSCh. 20 - Prob. 32PSCh. 20 - Prob. 33PSCh. 20 - Prob. 34PSCh. 20 - Prob. 35GQCh. 20 - Prob. 36GQCh. 20 - Prob. 37GQCh. 20 - Prob. 38GQCh. 20 - Prob. 40GQCh. 20 - Prob. 41ILCh. 20 - Prob. 42ILCh. 20 - Define the terms renewable and nonrenewable as...Ch. 20 - Prob. 44SCQCh. 20 - Prob. 45SCQCh. 20 - Prob. 46SCQCh. 20 - Prob. 47SCQCh. 20 - What is the likelihood that hydrogen (H2) will...Ch. 20 - Prob. 49SCQCh. 20 - Which sulfur compounds are atmospheric pollutants?...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each of the Followin, moleaks draw all OF The Resonance contributing stuluctures and compare these three molecules in terms of Resonance stabilization 1-C-1 a. b. H A-C+ О 112-1 C. F-C-F Farrow_forwarda. Explain Why electron withdrawing groupe tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures 6. Explain why -ll is an ortho -pura drccton evon though chlorine has a very High Electronegativityarrow_forwardC. Ν Harrow_forward
- a. H3C. N H3C CH3 HCNarrow_forwardол 2. восцапан (46:00) Curtius rearrangment 1. NaN3, heat -OHarrow_forwardQuestion 1. Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers.arrow_forward
- At an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. Hint: In this case you must choose the best answer to demonstrate the stereochemistry of H2 addition. 1.03 2. (CH3)2S BIZ CH₂OH 2. DMS KMnO4, NaOH ΖΗ Pd or Pt (catalyst) HBr 20 1 HBr ROOR (peroxide) HO H-SO HC 12 11 10 BH, THE 2. H2O2, NaOH Brz cold HI 19 18 17 16 MCPBA 15 14 13 A Br H₂O BH3⚫THF Brz EtOH Pd or Ni (catalyst) D₂ (deuterium) 1. Os04 2. H2O2 CH3CO3H (peroxyacid) 1. MCPBA 2. H₂O* H B + H H H "H C H H Darrow_forwardExplain how Beer’s Law can be used to determine the concentration in a selected food sample. Provide examples.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY