
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 25P
Eudesmol is a sesquiterpene found in eucalyptus. Propose a mechanism for its biosynthesis from farnesyl pyrophosphate.
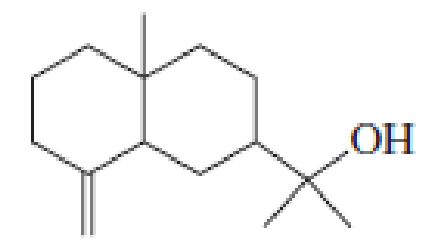
eudesmol
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the average mass of the 10 pennies? Report your value with correct significant figures.
What is the error (uncertainty) associated with each mass measurement due to the equipment?
What is the uncertainty associated with the average value? Note that the uncertainty of the balance will propagate throughout the calculation.
What is the standard deviation of the 10 mass measurements?
Explain the difference between the propagated uncertainty and the standard deviation. Which number would you use to describe the uncertainty in the measurement?
Calculate the total mass of the pennies with associated uncertainty.
Calculate the average density of a penny based on these data. Propagate the uncertainty values for both mass and volume in your calculations.
Can you help me and explain the answers please.
B 1 of 2
Additional problems in preparation to Midterm #1:
1.) How can the following compounds be prepared using Diels-Alder reaction:
CH3 O
CN
(a)
(b)
CN
CH3
2.) What is the missing reagent in the shown reaction?
H3C
+ ?
H3C
H3C
CN
H3C
''CN
(၁)
H
3.) Write the products 1,2-addition and 1,4-addition of DBr to 1,3-cyclohexadiene.
Remember, D is deuterium, a heavy isotope of hydrogen. It reacts exactly like hydrogen.
4.) In the shown reaction, which will be the kinetic product and which will be the
thermodynamic product?
H3C
CI
H3C
HCI
H3C
+
5.) Which of the following molecules is aromatic?
(a)
(b)
(c)
H
6.) Which of the following molecules is aromatic?
(a)
(b)
(c)
7.) Write the mechanism for the shown reaction.
+
Ха
AICI 3
CI
8.) Suggest reagents that would convert benzene into the shown compounds.
CI
NO2
-8-6-6-8-a
(a)
(b)
(c)
(d)
(e)
(a)
SO3H
Br
Chapter 20 Solutions
Essential Organic Chemistry, Global Edition
Ch. 20.1 - Prob. 1PCh. 20.2 - Prob. 2PCh. 20.2 - Prob. 3PCh. 20.2 - Draw the structure of an optically active fat...Ch. 20.4 - Prob. 6PCh. 20.4 - Prob. 7PCh. 20.4 - The membrane phospholipids in deer have a higher...Ch. 20.4 - Prob. 9PCh. 20.6 - Prob. 10PCh. 20.6 - Prob. 11P
Ch. 20.6 - Prob. 12PCh. 20.7 - Propose a mechanism for the biosynthesis of...Ch. 20.7 - Prob. 14PCh. 20.8 - Draw the individual 1,2-hydride and 1,2-methyl...Ch. 20.9 - Prob. 16PCh. 20 - Prob. 17PCh. 20 - Prob. 18PCh. 20 - Cardiolipins are found in heart muscles. Draw the...Ch. 20 - Prob. 20PCh. 20 - 5-Androstene-3,17-dione is isomerized to...Ch. 20 - Prob. 22PCh. 20 - Prob. 23PCh. 20 - Prob. 24PCh. 20 - Eudesmol is a sesquiterpene found in eucalyptus....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The number of 2sp^2 hybridized atoms in is: A. 8; B. 6; C.4; D.2; E.0;arrow_forwardThe highest boiling compound from among the following isA. 2-methylheptane; B. 3-methylheptane; C. 2,2-dimethylhexane;D. octane; E. 2,2,3-trimethylpentanearrow_forwardWhich of the following features are found in the most stable structure ofCH5NO that does not have a CO bond?w. a π bond, x. two NH bonds, y. one OH bond, z. 3 lone pairsA. w, x; B. x, y; C. y, z; D. x, y, z; E. all of them.arrow_forward
- Which one of the following functional groups is not present in thecompound shownA. amine; B. aldehyde, C. ether; D. amide. E. ketonearrow_forwardWhich of the following formulas correspond to at least one compound inwhich resonance is important?w. C2H5N x. C3H5Br; y. C3H4; z. C4H6.A. w, x, y; B. x, y, z; C. w, x, z; D. w, y, z; E. all of themarrow_forwardPredict the product(s) that are formed after each step for reactions 1-4. In each case, consider formation of any chiral center(s) and draw all expected stereoisomers. 1) OH 1) HBr (SN2) 2) NaOH, heat 3) BH3, THF 4) H2O2, NaOH 2) OH 1) SOCI 2, py 2) NaOEt 3) Br2, H₂O 3) OH 1) H2SO4 conc. 2) HBr, ROOR 3) KOtBu 4) OH 1) TsCl, py 2) NaOEt 3) 03 4) DMSarrow_forward
- Which of the following rings has the least strain in its most stableconformation?A. Cyclobutane; B. Cyclopentane; C. Cyclohexane; D. Cycloheptane;E. Cyclooctanearrow_forwardThe number of different carbon skeletons that have a main chain of 9carbons and an ethyl branch isA 3; B. 4; C. 5; D. 6; E. 7arrow_forwardQ5: Classify the following pair of molecules as constitutional isomers, enantiomers, diastereomers, the same molecule, or completely different molecules. Br O CI Br OH OH 111 Br .!!!/Br F OH and ...m Br Br OH CI Br OH ་་་་་" ། ་arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY