
Concept explainers
Interpretation:
For the given reaction, the reverse Diels-Alder reaction path has to be shown with Lewis structures of Butadiene sulfone and sulfuric acid.
Concept Introduction:
Diels-Alder reaction:
It is the reaction of conjugated dienes with double or triple bonded compounds which are known as “dienophiles”. The reaction is a
Example:
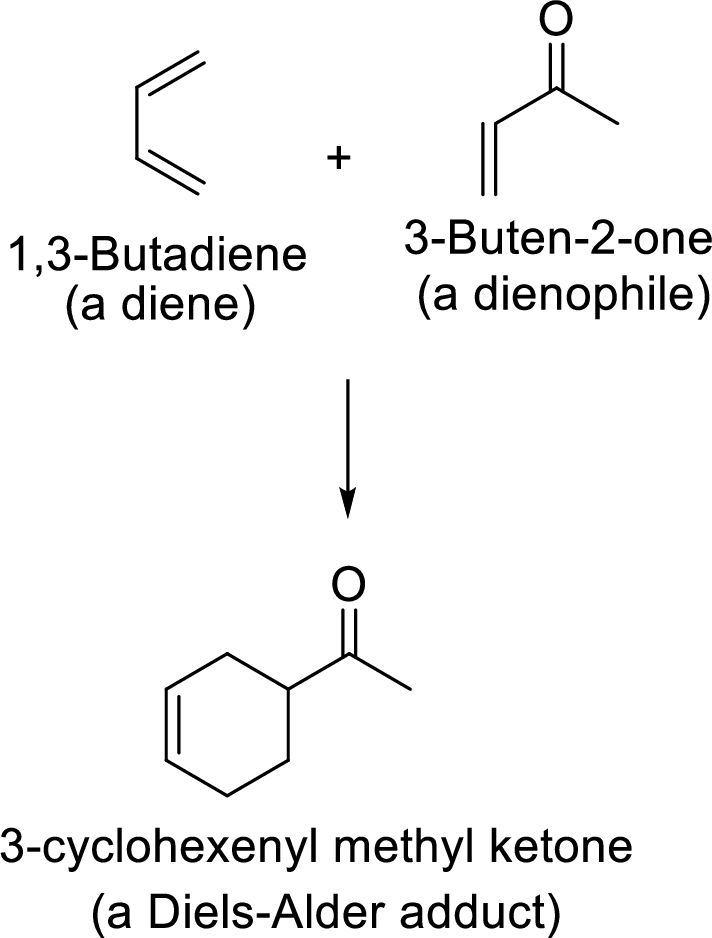
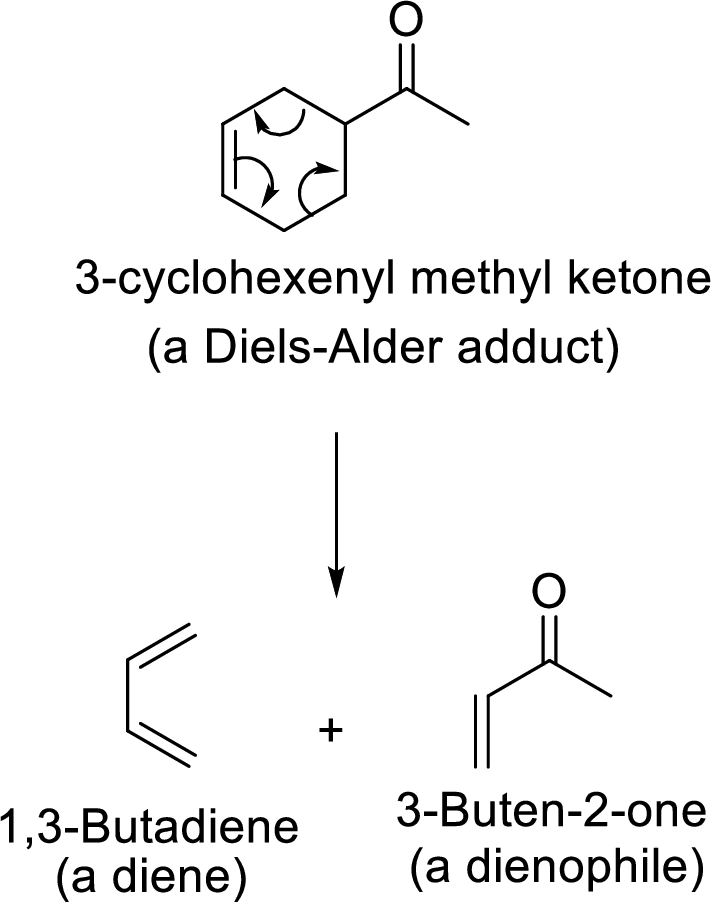
Reverse Diels-Alder reaction:
It is the reverse path of the Diels-Alder reaction in which the Diels-Alder adduct gives back its corresponding Diene and Dienophile as shown here:
Lewis dot structure: In the Lewis dot structure, an element will be represented by its elemental symbol. It will be surrounded by dots. Each dot represents the valence electron of that element. So the Lewis dot structure enables to represent an element with its valence electrons in
Trending nowThis is a popular solution!

Chapter 20 Solutions
EP ORGANIC CHEMISTRY-OWL V2 ACCESS
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
- Indicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol.arrow_forward2,2-Dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol. Indicate the products obtained.arrow_forward
- Add conditions above and below the arrow that turn the reactant below into the product below in a single transformationADS fint anditions 百 Abl res condinese NC ง Add on condtions 1.0 B H,N.arrow_forward3. Provide all the steps and reagents for this synthesis. OHarrow_forwardSteps and explanationarrow_forward
- Steps and explanations please.arrow_forwardSteps on how to solve. Thank you!arrow_forward3. Name this ether correctly. H₁C H3C CH3 CH3 4. Show the best way to make the ether in #3 by a Williamson Ether Synthesis. Start from an alcohol or phenol. 5. Draw the structure of an example of a sulfide.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

