
(a)
Interpretation:
The product which is formed by the reaction of
Concept introduction:
The curved-arrow notation is used to show the transfer of electrons from one atom to another. The curved arrow has two barbs (head and tail) which represent the direction of electron flow.
Answer to Problem 20.31AP
The curved arrow notation and product for the reaction of
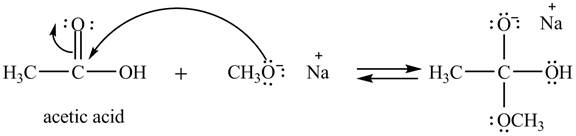
Explanation of Solution
The treatment of sodium methoxide with a
In the first step, the electron pairs of oxygen present in methoxide ion attacks the acyl carbon atom of acetic acid and bonding pair of electrons present in the pi bond of acyl group shifts towards oxygen atom simultaneously. Due this an intermediate is formed as shown below.
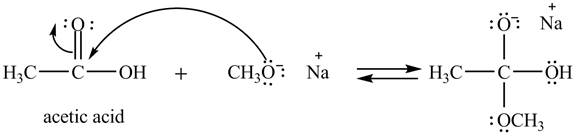
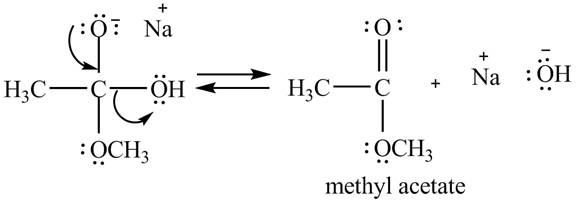
Figure 1
In the second step, the regeneration of the acyl group takes place by the movement of electron pair of negative charged oxygen atom and also the removal of hydroxide ion takes place from the intermediate simultaneously. Hydroxide ion gets removed by grabbing the electron pair of

Figure 2
Therefore, methyl acetate is desired product formed by the reaction of
The curved arrow notation and product for the reaction of
(b)
Interpretation:
The product which is formed by the reaction of
Concept introduction:
The curved-arrow notation is used to show the transfer of electrons from one atom to another. The curved arrow has two barbs (head and tail) which represent the direction of electron flow.
Answer to Problem 20.31AP
The curved arrow notation and product for the reaction of ![]() of acetic acid are shown below.
of acetic acid are shown below.
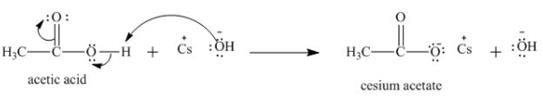
Explanation of Solution
The treatment of a base like
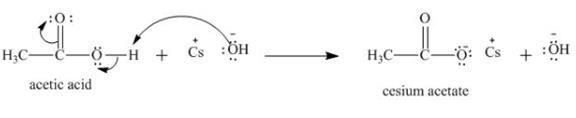
Figure 3
Therefore, cesium acetate is desired product formed by the reaction of ![]() of
of
The curved arrow notation and product for the reaction of
(c)
Interpretation:
The product which is formed by the reaction of ![]() of acetic acid and the corresponding curved-arrow notation are to be stated.
of acetic acid and the corresponding curved-arrow notation are to be stated.
Concept introduction:
The curved-arrow notation is used to show the transfer of electrons from one atom to another. The curved arrow has two barbs (head and tail) which represent the direction of electron flow.
Answer to Problem 20.31AP
The curved arrow notation and product for the reaction of
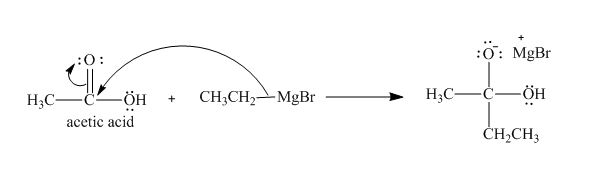
Explanation of Solution
The treatment of Grignard reagent with a carboxylic acid results into a nucleophilic substitution reaction. The steps for the formation of the product of the reaction between
In the first step, the bonding electron pairs of

Figure 4
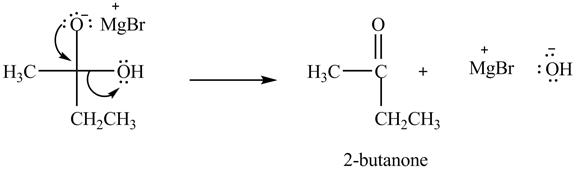
In the second step, the regeneration of the acyl group takes place by the movement of electron pair of negative charged oxygen atom and also the removal of hydroxide ion takes place from the intermediate simultaneously. Hydroxide ion gets removed by grabbing the electron pair of

Figure 5
Therefore, ![]() of
of
The curved arrow notation and product for the reaction of
(d)
Interpretation:
The product which is formed by the reaction of
Concept introduction:
The curved-arrow notation is used to show the transfer of electrons from one atom to another. The curved arrow has two barbs (head and tail) which represent the direction of electron flow.
Answer to Problem 20.31AP
The curved arrow notation and product for the reaction of
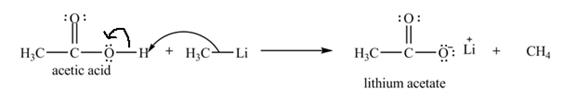
Explanation of Solution
The treatment of a base like

Figure 6
Therefore, lithium acetate is desired product formed by the reaction of
The curved arrow notation and product for the reaction of
(e)
Interpretation:
The product which is formed by the reaction of
Concept introduction:
The curved-arrow notation is used to show the transfer of electrons from one atom to another. The curved arrow has two barbs (head and tail) which represent the direction of electron flow.
Answer to Problem 20.31AP
The curved arrow notation and product for the reaction of
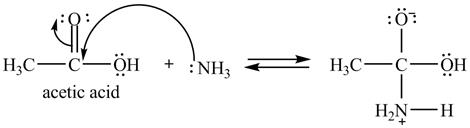
Explanation of Solution
The treatment of ammonia with a carboxylic acid results into a nucleophilic acyl substitution reaction. The steps for the formation of the product of the reaction between
In the first step, the lone of pair of electrons of
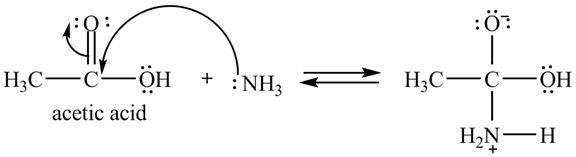
Figure 7
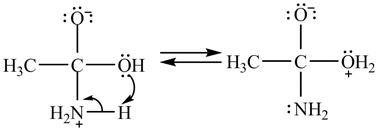
In the second step, electron pairs of oxygen in
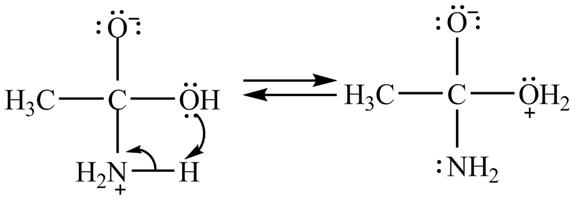
Figure 8
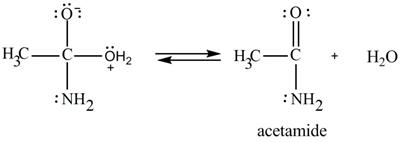
In the third step, the regeneration of the acyl group takes place by the movement of electron pair of negative charged oxygen atom and also the removal of hydroxide ion takes place from the intermediate simultaneously. Hydroxide ion gets removed by grabbing the electron pair of
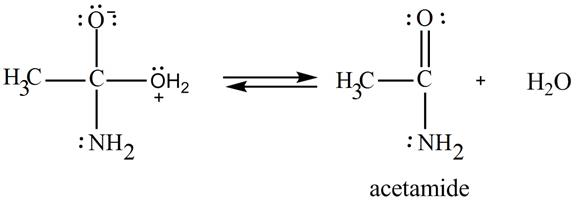
Figure 9
Therefore,
The curved arrow notation and product for the reaction of
(f)
Interpretation:
The product which is formed by the reaction of
Concept introduction:
The curved-arrow notation is used to show the transfer of electrons from one atom to another. The curved arrow has two barbs (head and tail) which represent the direction of electron flow.
Answer to Problem 20.31AP
The curved arrow notation and product for the reaction of
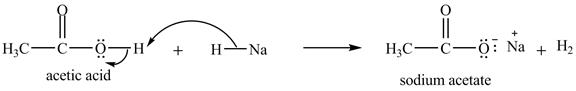
Explanation of Solution
The treatment of a base like
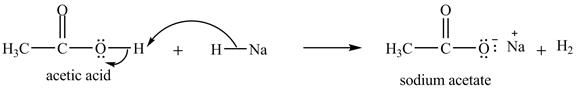
Figure 10
Therefore, sodium acetate is desired product formed by the reaction of
The curved arrow notation and product for the reaction of
(g)
Interpretation:
The product which is formed by the reaction of
Concept introduction:
The curved-arrow notation is used to show the transfer of electrons from one atom to another. The curved arrow has two barbs (head and tail) which represent the direction of electron flow.
Answer to Problem 20.31AP
The curved arrow notation and product for the reaction of
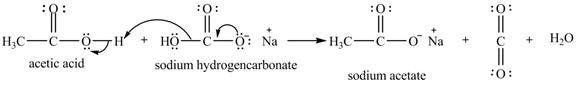
Explanation of Solution
The given compound is shown below.
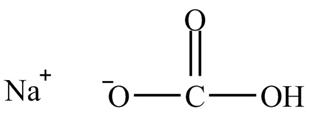
Figure 11
The given compound is sodium hydrogencarbonate. The treatment of a base like sodium hydrogencarbonate with acetic acid produces a salt known as sodium acetate. In the first step of the reaction between
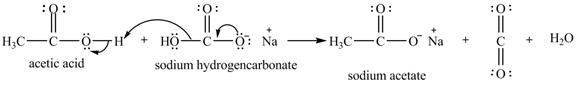
Figure 12
Therefore, sodium acetate is desired product formed by the reaction of
The curved arrow notation and product for the reaction of
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry
- Why is analysing salt content (using Mohr titration) in both regular & salt reduced tomato sauce important?arrow_forwardIn the image below, correctly name the glassware # _P ( Blank 1) and T ( Blank 2). 景 A W Blank # 1 Blank #2 1000 +19 E E D 0 0-0 G H A A K Π 12 R M N S 0-0-arrow_forwardFeedback: Your answer is incorrect. Predict the major products of the following organic reaction: CN Δ + A ? NC Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. esc Check 80 MH F1 F2 F3 F4 F5 50 @ # C % 95 € Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C A DII F6 F7 F8 7 * 8 Λ & 6 F9 F10 9 0 4arrow_forward
- Incorrect Feedback: Your answer is incorrect. Predict the major products of the following organic reaction: ཤིགས་བྱ རྩ་ཅད་ཀྱིས་༢༩ + Some important notes: A ^ ? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. E Check 0 لا Save For La ©2025 McGraw Hill LLC. All Rights Reserved. Terms of All F9 Aarrow_forwardPredict the major products of the following organic reaction: + Δ A ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privaarrow_forwardesc 2 Incorrect Feedback: Your answer is incorrect. Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? A O • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. . If your answer is no, check the box under the drawing area instead. Check F1 ! @ X C Save For Later Submit Assignment 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 80 et A ད 1 4 F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 # $ 45 % A 6 87 & * 8 9 ) 0 + ||arrow_forward
- Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ?A Δ O • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilit ku F11arrow_forward१ eq ine teaching and × + rn/takeAssignment/takeCovalentActivity.do?locator-assignment-take [Review Topics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. The IUPAC name is In progress mit Answer Retry Entire Group 5 more group attempts remaining Cengage Learning | Cengage Technical Support Save and Exitarrow_forwardDraw the molecules.arrow_forward
- Draw the mechanism for the acid-catalyzed dehydration of 2-methyl-hexan-2-ol with arrows please.arrow_forward. Draw the products for addition reactions (label as major or minor) of the reaction between 2-methyl-2-butene and with following reactants : Steps to follow : A. These are addition reactions you need to break a double bond and make two products if possible. B. As of Markovnikov rule the hydrogen should go to that double bond carbon which has more hydrogen to make stable products or major product. Here is the link for additional help : https://study.com/academy/answer/predict-the-major-and-minor-products-of-2-methyl- 2-butene-with-hbr-as-an-electrophilic-addition-reaction-include-the-intermediate- reactions.html H₂C CH3 H H3C CH3 2-methyl-2-butene CH3 Same structure CH3 IENCESarrow_forwardDraw everything on a piece of paper including every single step and each name provided using carbons less than 3 please.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
