
Concept explainers
(a)
Interpretation: The orbitals are used to form the indicated bonds in
Concept introduction:
Answer to Problem 20.1P
The orbitals are used to form bond
Explanation of Solution
The given structure of
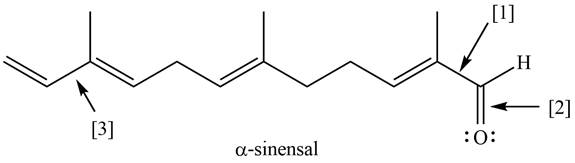
Figure 1
Bond
Bond
Bond
The orbitals are used to form bond
(b)
Interpretation: The type of orbitals where the lone pairs on
Concept introduction:
Answer to Problem 20.1P
The type of orbitals where the lone pairs on
Explanation of Solution
The oxygen atom of carbonyl carbon in
The type of orbitals where the lone pairs on
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
