
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 20.65P
Synthesize each compound from cyclohexanol using any other organic or inorganic compounds.
a.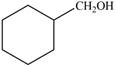 c.
c.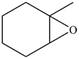 e.
e.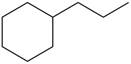 g.
g.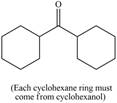
b.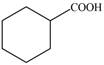 d.
d. 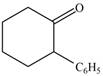 f.
f.![]() h.
h.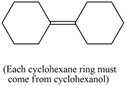
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
State two variables on which the transport number in electrochemistry depends.
Among the methods for measuring transport numbers are the Hittorf method and the moving surface method. Let's explain them briefly.
can you help me solve and highlight these hw
Chapter 20 Solutions
Organic Chemistry
Ch. 20 - Prob. 20.1PCh. 20 - Which carbonyl groups in the anticancer drug taxol...Ch. 20 - Prob. 20.3PCh. 20 - Prob. 20.4PCh. 20 - Problem 20.5 What aldehyde or ketone is needed to...Ch. 20 - Prob. 20.6PCh. 20 - Problem 20.7 Draw the products formed when is...Ch. 20 - Prob. 20.8PCh. 20 - Prob. 20.9PCh. 20 - Prob. 20.10P
Ch. 20 - Draw the structure of both an acid chloride and an...Ch. 20 - Problem 20.12 Draw the products formed from ...Ch. 20 - Prob. 20.13PCh. 20 - Prob. 20.14PCh. 20 - What product is formed when...Ch. 20 - Prob. 20.16PCh. 20 - Problem-20.16 Review the oxidation reactions using...Ch. 20 - Problem-20.17 Write the step(s) needed to convert ...Ch. 20 - Problem-20.18 Oct-1-yne reacts rapidly with ,...Ch. 20 - Draw the product formed when each organometallic...Ch. 20 - Draw the product of each reaction. a.c. b.d.Ch. 20 - Draw the products including stereochemistry of the...Ch. 20 - What Grignard reagent and carbonyl compound are...Ch. 20 - Problem 20.24 Linalool (the Chapter 9 opening...Ch. 20 - Problem 20.25 What Grignard reagent and carbonyl...Ch. 20 - Prob. 20.26PCh. 20 - Draw the products formed when each compound is...Ch. 20 - What ester and Grignard reagent are needed to...Ch. 20 - What organocuprate reagent is needed to convert...Ch. 20 - What reagent is needed to convert (CH3)2CHCH2CHO...Ch. 20 - Prob. 20.31PCh. 20 - What carboxylic acid formed from each alkyl halide...Ch. 20 - Prob. 20.33PCh. 20 - Prob. 20.34PCh. 20 - Problem 20.35 Synthesize each compound from...Ch. 20 - Prob. 20.36PCh. 20 - 20.37 Devise a synthesis of each alcohol from...Ch. 20 - 20.38 Draw the products formed when pentanal is...Ch. 20 - Prob. 20.39PCh. 20 - Draw the product formed when CH3CH2CH2MgBr is...Ch. 20 - Draw the product formed when (CH3CH2CH2CH2)2CuLi...Ch. 20 - The stereochemistry of the products of reduction...Ch. 20 - Prob. 20.43PCh. 20 - What reagent is needed to convert...Ch. 20 - What reagent is needed to convert...Ch. 20 - Draw the products or each reduction reaction. a....Ch. 20 - Prob. 20.47PCh. 20 - Draw all stereoisomers formed in each reaction. a....Ch. 20 - Prob. 20.49PCh. 20 - 20.46 Treatment of ketone A with ethynylithium...Ch. 20 - 20.47 Explain why metal hydride reduction gives an...Ch. 20 - Prob. 20.52PCh. 20 - 20.49 Identify the lettered compounds in the...Ch. 20 - Prob. 20.54PCh. 20 - Draw a stepwise mechanism for each reaction. a. b.Ch. 20 - Prob. 20.56PCh. 20 - 20.54 Draw a stepwise mechanism for the following...Ch. 20 - What Grignard reagent and aldehyde or ketone are...Ch. 20 - Prob. 20.59PCh. 20 - What ester and Grignard reagent are needed to...Ch. 20 - What organolithium reagent and carbonyl compound...Ch. 20 - 20.59 What epoxide and organometallic reagent are...Ch. 20 - Prob. 20.63PCh. 20 - 20.61 Propose two different methods to synthesize...Ch. 20 - Synthesize each compound from cyclohexanol using...Ch. 20 - Prob. 20.66PCh. 20 - Prob. 20.67PCh. 20 - Devise three different methods to prepare each...Ch. 20 - Convert benzene into each compound. You may also...Ch. 20 - Design a synthesis of each compound from alcohols...Ch. 20 - Synthesize each compound from the given starting...Ch. 20 - 20.69 An unknown compound A (molecular formula )...Ch. 20 - 20.70 Treatment of compound C (molecular formula )...Ch. 20 - 20.71 Treatment of compound E (molecular formula )...Ch. 20 - 20.72 Reaction of butanenitrile () with methyl...Ch. 20 - 20.73 Treatment of isobutene with forms a...Ch. 20 - Prob. 20.77PCh. 20 - Prob. 20.78PCh. 20 - 20.76 Lithium tri-sec-butylborohydride, also known...Ch. 20 - Prob. 20.80PCh. 20 - Prob. 20.81PCh. 20 - Prob. 20.82PCh. 20 - 20.80 Draw a stepwise mechanism for the following...Ch. 20 - Prob. 20.84P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 10. Stereochemistry. Assign R/S stereochemistry for the chiral center indicated on the following compound. In order to recieve full credit, you MUST SHOW YOUR WORK! H₂N CI OH CI カー 11. () Stereochemistry. Draw all possible stereoisomers of the following compound. Assign R/S configurations for all stereoisomers and indicate the relationship between each as enantiomer, diastereomer, or meso. NH2 H HNH, -18arrow_forwardb) 8. Indicate whether the following carbocation rearrangements are likely to occur Please explain your rational using 10 words or less not likely to occur • The double bond is still in the Same position + Likely to oc occur WHY? -3 H3C Brave Chair Conformers. Draw the chair conformer of the following substituted cyclohexane. Peform a RING FLIP and indicate the most stable conformation and briefly explain why using 20 words or less. CI 2 -cobs ?? MUST INDICATE H -2 -2 Br EQ Cl OR AT Br H& most stable WHY? - 4arrow_forwardCH 12 Conformational Analysis. Draw all 6 conformers (one above each letter) of the compound below looking down the indicated bond. Write the letter of the conformer with the HIGHEST and LOWEST in energies on the lines provided. NOTE: Conformer A MUST be the specific conformer of the structure as drawn below -4 NOT HOH OH 3 Conformer A: Br OH A Samo Br H 04 Br H H3 CH₂ H anti stagere Br CH clipsed H Brott H IV H MISSING 2 -2 B C D E F X 6 Conformer with HIGHEST ENERGY: 13. (1 structure LOWEST ENERGY: Nomenclature. a) Give the systematic (IUPAC) name structure. b) Draw the corresponding to this name. HINT: Do not forget to indicate stereochemistry when applicable. a) ८८ 2 "Br {t༐B,gt)-bemn€-nehpརི་ཚ༐lnoa Parent name (noname) 4 Bromo Sub = 2-methylethyl-4 Bromo nonane b) (3R,4S)-3-chloro-4-ethyl-2,7-dimethyloctane # -2 -2arrow_forward
- in the scope of the SCH4U course! please show all steps as im still learning how to format my answers in the format given, thank you!arrow_forwardhelp me solve this HWarrow_forwardMolecules of the form AH2 can exist in two potential geometries: linear or bent. Construct molecular orbital diagrams for linear and bent CH2. Identify the relevant point group, include all of the appropriate symmetry labels and pictures, and fill in the electrons. Which geometry would you predict to be more stable, and why? (Please draw out the diagram and explain)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY