
Concept explainers
What is meant by the term “unsaturated hydrocarbon”? What structural feature characterizes
Interpretation:
The meaning of “unsaturated hydrocarbon” should be determined along with the structural features which characterizes unsaturated hydrocarbons.
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon
Answer to Problem 1ALQ
The hydrocarbons in which carbon-carbon multiple bonds are present is said to be unsaturated hydrocarbons.
The unsaturated hydrocarbon which contains one or more double bonds in the structure is known as alkene whereas which contains one or more triple bonds in the structure is known as alkyne.
Explanation of Solution
Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
Unsaturated hydrocarbon which contains one or more double bond is known as alkenes and which contains one or more triple bonds are said to be alkynes.
Carbon atoms linked with double bond are bound with each other by one sigma and one pi bond and carbon atoms linked with triple bond are bound with each other by one sigma and two pi bonds.
For example:
The structure of alkene and alkyne is given as:
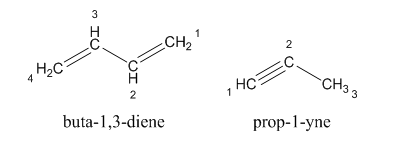
The hydrocarbons in which carbon-carbon multiple bonds are present is said to be unsaturated hydrocarbons.
The unsaturated hydrocarbon which contains one or more double bonds in the structure is known as alkene whereas which contains one or more triple bonds in the structure is known as alkyne.
Want to see more full solutions like this?
Chapter 20 Solutions
Introductory Chemistry: A Foundation
Additional Science Textbook Solutions
Microbiology: Principles and Explorations
College Physics: A Strategic Approach (3rd Edition)
Brock Biology of Microorganisms (15th Edition)
SEELEY'S ANATOMY+PHYSIOLOGY
Organic Chemistry (8th Edition)
- Identify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forward
- Rank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forward
- Why isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forward
- Complete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forwardQ Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





