
Concept explainers
Interpretation:
The structural formula for 2-propanol should be drawn and whether it is primary, secondary, or tertiary alcohol should be indicated.
Concept Introduction:
The formula in which the arrangements of atoms in a molecule are shown is said to be the structural formula.
- An organic compound in which hydroxyl
functional group that is -OH is bonded to the carbon atom is said to be an alcohol. The general formula for alcohol is Cn H2 n + 1 OH. Based on the attachment to the carbon the alcohols are classified as primary, secondary, or tertiary alcohol.
When alcohol (having hydroxyl group) is attached to a primary carbon atom (carbon atom to which one carbon atom is attached) then such alcohol is said to be a primary alcohol.
When alcohol (having hydroxyl group) is attached to a secondary carbon atom (carbon atom to which two carbon atoms are attached) then such alcohol is said to be a secondary alcohol.
When alcohol (having hydroxyl group) is attached to a tertiary carbon atom (carbon atom to which three carbon atoms are attached) then such alcohol is said to be a tertiary alcohol.
Answer to Problem 137AP
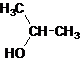
Secondary alcohol.
Explanation of Solution
For number of carbons atoms in chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
The numbering of the carbon chain is done in such a way that the substituents get the lower number.
The given name is 2-propanol that means the parent chain has 3 number of carbons in the chain and suffix -ol represents it is an alcohol group that is R-OH (where R is
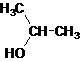
Since, -OH is attached to secondary carbon so, it is a secondary alcohol.
Interpretation:
The structural formula for 2-methyl-2-propanol should be drawn and whether it is primary, secondary, or tertiary alcohol should be indicated.
Concept Introduction:
The formula in which the arrangements of atoms in a molecule are shown is said to be the structural formula.
- An organic compound in which hydroxyl functional group that is -OH is bonded to the carbon atom is said to be an alcohol. The general formula for alcohol is Cn H2 n + 1 OH. Based on the attachment to the carbon the alcohols are classified as primary, secondary, or tertiary alcohol.
When alcohol (having hydroxyl group) is attached to a primary carbon atom (carbon atom to which one carbon atom is attached) then such alcohol is said to be a primary alcohol.
When alcohol (having hydroxyl group) is attached to a secondary carbon atom (carbon atom to which two carbon atoms are attached) then such alcohol is said to be a secondary alcohol.
When alcohol (having hydroxyl group) is attached to a tertiary carbon atom (carbon atom to which three carbon atoms are attached) then such alcohol is said to be a tertiary alcohol.
Answer to Problem 137AP
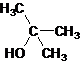
Tertiary alcohol.
Explanation of Solution
For number of carbons atoms in chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
The numbering of the carbon chain is done in such a way that the substituents get the lower number.
The given name is 2-methyl-2-propanol that means the parent chain has 3 number of carbons in the chain and suffix -ol represents it is an alcohol group that is R-OH (where R is aryl/alkyl group) at 2 position, and a methyl substitution at 2 position. So, the structural formula of 2-methyl-2-propanol is:
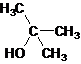
Since, -OH is attached to tertiary carbon so, it is a tertiary alcohol.
Interpretation:
The structural formula for 4-isopropyl-2-heptanol should be drawn and whether the it is primary, secondary, or tertiary alcohol should be indicated.
Concept Introduction:
The formula in which the arrangements of atoms in a molecule are shown is said to be the structural formula.
- An organic compound in which hydroxyl functional group that is -OH is bonded to the carbon atom is said to be an alcohol. The general formula for alcohol is Cn H2 n + 1 OH. Based on the attachment to the carbon the alcohols are classified as primary, secondary, or tertiary alcohol.
When alcohol (having hydroxyl group) is attached to a primary carbon atom (carbon atom to which one carbon atom is attached) then such alcohol is said to be a primary alcohol.
When alcohol (having hydroxyl group) is attached to a secondary carbon atom (carbon atom to which two carbon atoms are attached) then such alcohol is said to be a secondary alcohol.
When alcohol (having hydroxyl group) is attached to a tertiary carbon atom (carbon atom to which three carbon atoms are attached) then such alcohol is said to be a tertiary alcohol.
Answer to Problem 137AP
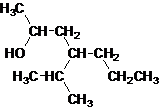
Secondary alcohol.
Explanation of Solution
For number of carbons atoms in chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
The numbering of the carbon chain is done in such a way that the substituents gets the lower number.
The given name is 4-isopropyl-2-heptanol that means the parent chain has 7 number of carbons in the chain and suffix -ol represents it is an alcohol group that is R-OH (where R is aryl/alkyl group) at 2 position, and anisopropyl substitution at 4 position. So, the structural formula of 4-isopropyl-2-heptanol is:
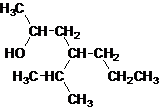
Since, -OH is attached to secondary carbon so, it is a secondary alcohol.
Interpretation:
The structural formula for 2, 3-dichloro-1-pentanol should be drawn and whether the it is primary, secondary, or tertiary alcohol should be indicated.
Concept Introduction:
The formula in which the arrangements of atoms in a molecule are shown is said to be the structural formula.
- An organic compound in which hydroxyl functional group that is -OH is bonded to the carbon atom is said to be an alcohol. The general formula for alcohol is Cn H2 n + 1 OH. Based on the attachment to the carbon the alcohols are classified as primary, secondary, or tertiary alcohol.
When alcohol (having hydroxyl group) is attached to a primary carbon atom (carbon atom to which one carbon atom is attached) then such alcohol is said to be a primary alcohol.
When alcohol (having hydroxyl group) is attached to a secondary carbon atom (carbon atom to which two carbon atoms are attached) then such alcohol is said to be a secondary alcohol.
When alcohol (having hydroxyl group) is attached to a tertiary carbon atom (carbon atom to which three carbon atoms are attached) then such alcohol is said to be a tertiary alcohol.
Answer to Problem 137AP
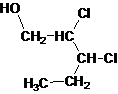
Primary alcohol.
Explanation of Solution
For number of carbons atoms in chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
The numbering of the carbon chain is done in such a way that the substituents get the lower number.
The given name is 2, 3-dichloro-1-pentanol that means the parent chain has 5 number of carbons in the chain and suffix -ol represents it is an alcohol group that is R-OH (where R is aryl/alkyl group) at 1 position, and 2 chlorine substitution at 2 and 3 position. So, the structural formula of 2, 3-dichloro-1-pentanol is:
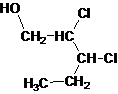
Since, -OH is attached to primary carbon so, it is a primary alcohol.
Want to see more full solutions like this?
Chapter 20 Solutions
Bundle: Introductory Chemistry: A Foundation, 8th + OWLv2 6-Months Printed Access Card
- Draw the most stable cations formed in the mass spectrometer by a deavage of the following compound Draw the most stable cations formed in the mass spectrometer by a cleavage of the following compound онarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting anand product sytucutrs, draw the curved electron-pusing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bind-making stepsarrow_forwardDraw the major elimination and substitution products formed in this reavtion. Use a dash or wedge bond to indicatr the stereochemistry of substituents on assymetric centers, wheere applicable. Ignore any inorganic byproducts.arrow_forward
- Draw the two possible products produced in this E2 elimination. Ignore any inorganic byproductsarrow_forwardDraw the major products of this SN1 reaction. Ignore any inorganic byproducts.arrow_forwardDraw the major elimination and substitution products formed in this reaction. Use a dash or wedge bond to indicate the stereochemistry of substituents on asymmetric centers, wehre applicable. Ignore and inorganic byproducts.arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Drawing Arrows THE Problem 33 of 35 N. C:0 Na + Submit Drag To Pan +arrow_forwardDraw the product of the E2 reaction shown below. Include the correct stereochemistry. Ignore and inorganic byproducts.arrow_forwardDraw the major producrs of this SN1 reaction. Ignore any inorganic byproducts. Use a dash or wedge bond to indicate the sereochemistry of substituents on asymmetric centers where appllicable.arrow_forward
- 5) Oxaloacetic Acid is an important intermediate in the biosynthesis of citric acid. Synthesize oxaloacetic acid using a mixed Claisen Condensation reaction with two different esters and a sodium ethoxide base. Give your answer as a scheme Hint 1: Your final acid product is producing using a decarboxylation reaction. Hint 2: Look up the structure of oxalic acid. HO all OH oxaloacetic acidarrow_forward20. The Brusselator. This hypothetical system was first proposed by a group work- ing in Brussels [see Prigogine and Lefever (1968)] in connection with spatially nonuniform chemical patterns. Because certain steps involve trimolecular reac tions, it is not a model of any real chemical system but rather a prototype that has been studied extensively. The reaction steps are A-X. B+X-Y+D. 2X+ Y-3X, X-E. 305 It is assumed that concentrations of A, B, D, and E are kept artificially con stant so that only X and Y vary with time. (a) Show that if all rate constants are chosen appropriately, the equations de scribing a Brusselator are: dt A-(B+ 1)x + x²y, dy =Bx-x²y. diarrow_forwardProblem 3. Provide a mechanism for the following transformation: H₂SO A Me. Me Me Me Mearrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





