
Concept explainers
An
- (a) Identify the element and give its symbol.
- (b) Give the element’s
atomic number . - (c) Give the mass number of the isotope.
- (d) This element has two naturally occurring isotopes. Given the information in the table, calculate the atomic weight of the element.
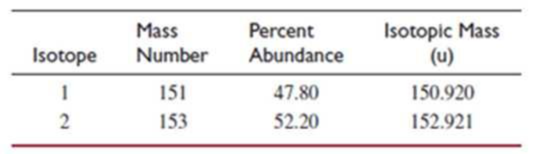
- (e) In which region of the periodic table is the element found? Explain your answer.
- (f) Is the element a metal, metalloid, or nonmetal? Explain your answer.
- (g) This element, used in compact fluorescent light bulbs and computer screens, has an atomic radius of 180 pm. Calculate how long the chain of atoms would be if all the atoms in a 1.25-mg sample of this element were put into a row.
(a)
Interpretation:
The isotope of an element contains 63 protons and 91 neutrons. The element has to be identified and the symbol of the element has to be given.
Explanation of Solution
Number of protons of the element is 63.
Number of neutrons of the element is 91.
The atomic number is same as that of number of protons, 63. Also the number of protons is same as that number of electrons in an uncharged atom. The element is Europium with a symbol of
(b)
Interpretation:
The atomic number of element with 63 protons and 91 neutrons has to be given.
Explanation of Solution
Number of protons of the element is 63.
The atomic number is same as that of number of protons, 63.
Atomic number
(c)
Interpretation:
The mass number of the isotope with 63 protons and 91 neutrons has to be given.
Explanation of Solution
Number of protons of the element is 63.
Number of neutrons of the element is 91.
Atomic number
Mass number is the sum of atomic number of the element and number of neutrons.
Isotope’s mass number
(d)
Interpretation:
The element has two naturally occurring isotopes. From the table given the atomic weight of the element has to be calculated.
Explanation of Solution
Every 10000 atoms of the element contain 4780 atoms of the
(e)
Interpretation:
The region in which the element is found in periodic table has to be explained.
Explanation of Solution
The element is found in the lanthanide series because it shows up in the row labeled “Lanthanides”.
(f)
Interpretation:
The given element is a metal, metalloid or non-metal has to be found and the answer has to be explained.
Explanation of Solution
The element Europium is a transition metal.
(g)
Interpretation:
The element given has an atomic radius of
Explanation of Solution
The atomic radius is
Want to see more full solutions like this?
Chapter 2 Solutions
Chemistry: The Molecular Science
- Draw the Michael Adduct and the final product of the Robinson annulation reaction. Ignore inorganic byproducts.arrow_forwardDraw the Michael adduct and final product of the Robinson annulation reaction. Ignore inorganic byproductsarrow_forwardPost Lab Questions. 1) Draw the mechanism of your Diels-Alder cycloaddition. 2) Only one isomer of product is formed in the Diels-Alder cycloaddition. Why? 3) Imagine that you used isoprene as diene - in that case you don't have to worry about assigning endo vs exo. Draw the "endo" and "exo" products of the Diels-Alder reaction between isoprene and maleic anhydride, and explain why the distinction is irrelevant here. 4) This does not hold for other dienes. Draw the exo and endo products of the reaction of cyclohexadiene with maleic anhydride. Make sure you label your answers properly as endo or exo. 100 °C Xylenes ??? 5) Calculate the process mass intensity for your specific reaction (make sure to use your actual amounts of reagent).arrow_forward
- Indicate the product(s) A, B C and D that are formed in the reaction: H + NH-NH-CH [A+B] [C+D] hydrazonesarrow_forwardHow can you prepare a 6 mL solution of 6% H2O2, if we have a bottle of 30% H2O2?arrow_forwardHow many mL of H2O2 from the 30% bottle must be collected to prepare 6 mL of 6% H2O2.arrow_forward
- Indicate the product(s) B and C that are formed in the reaction: HN' OCH HC1 B + mayoritario C minoritario OCH3arrow_forwardIndicate the product(s) that are formed in the reaction: NH-NH, OCH3 -H₂O OCH3arrow_forward21.38 Arrange the molecules in each set in order of increasing acidity (from least acidic to most acidic). OH OH SH NH2 8 NH3 OH (b) OH OH OH (c) & & & CH3 NO2 21.39 Explain the trends in the acidity of phenol and the monofluoro derivatives of phenol. OH OH OH OH PK 10.0 PK 8.81 PK 9.28 PK 9.81arrow_forward
- identify which spectrum is for acetaminophen and which is for phenacetinarrow_forwardThe Concept of Aromaticity 21.15 State the number of 2p orbital electrons in each molecule or ion. (a) (b) (e) (f) (c) (d) (h) (i) DA (k) 21.16 Which of the molecules and ions given in Problem 21.15 are aromatic according to the Hückel criteria? Which, if planar, would be antiaromatic? 21.17 Which of the following structures are considered aromatic according to the Hückel criteria? ---0-0 (a) (b) (c) (d) (e) (h) H -H .8.0- 21.18 Which of the molecules and ions from Problem 21.17 have electrons donated by a heteroatom?arrow_forward1. Show the steps necessary to make 2-methyl-4-nonene using a Wittig reaction. Start with triphenylphosphine and an alkyl halide. After that you may use any other organic or inorganic reagents. 2. Write in the product of this reaction: CH3 CH₂ (C6H5)₂CuLi H₂O+arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





