
EBK ESSENTIAL ORGANIC CHEMISTRY
3rd Edition
ISBN: 8220100659461
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 54P
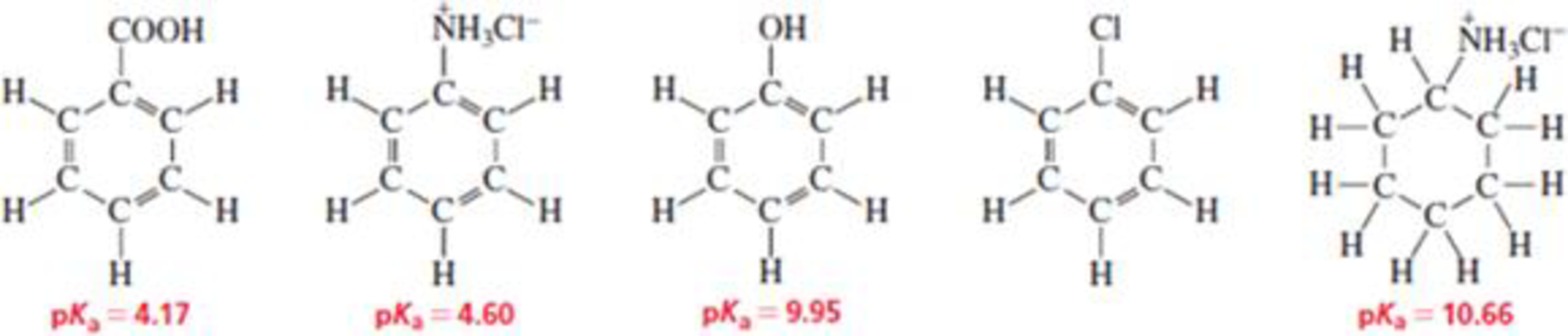
How could you separate a mixture of the following compounds? The reagents available to you are water, ether. 1.0 M HCl, and 1.0 M NaOH. (Hint: Sec Problem 34.)
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Don't used hand raiting and don't used Ai solution
Don't used hand raiting and don't used Ai solution
2.
200
LOD
For an unknown compound with a molecular ion of 101 m/z:
a.
Use the molecular ion to propose at least two molecular formulas. (show your work)
b.
What is the DU for each of your possible formulas? (show your work)
C.
Solve the structure and assign each of the following spectra.
8
6
4
2
(ppm)
150
100
50
ō (ppm)
4000
3000
2000
1500
1000
500
HAVENUMBERI-11
Chapter 2 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
Ch. 2.1 - Which of the following are not acids? CH3COOH CO2...Ch. 2.1 - Draw the products of the acidbase reaction when a....Ch. 2.1 - a.What is the conjugate acid of each of the...Ch. 2.2 - a. Which is a stronger acid, one with a pKa of 5.2...Ch. 2.2 - Prob. 5PCh. 2.2 - Antacids are compounds that neutralize stomach...Ch. 2.2 - Are the following body fluids acidic or basic? a....Ch. 2.3 - Draw the conjugate acid of each of the following:...Ch. 2.3 - a. Write an equation showing CH3OH reacting as an...Ch. 2.3 - Prob. 10P
Ch. 2.3 - a. Which is a stronger base, CH3COO or HCOO? (The...Ch. 2.3 - Using the pKa values in Section 2.3, rank the...Ch. 2.4 - Prob. 13PCh. 2.5 - Prob. 14PCh. 2.5 - Ethyne has a pKa value of 25, water has a pKa...Ch. 2.5 - Which of the following bases can remove a proton...Ch. 2.6 - List the ions (CH3, NH2, HO, and F) in order from...Ch. 2.6 - List the carbanions shown in the margin in order...Ch. 2.6 - Which is a stronger acid?Ch. 2.6 - a. Draw the products of the following reactions: A...Ch. 2.6 - List the halide ions (F, Cl, Br, and I) in order...Ch. 2.6 - a. Which is more electronegative, oxygen or...Ch. 2.6 - Which is a stronger acid? a. HCl or HBr b....Ch. 2.6 - a. Which of the halide ions (F, Cl, Br, and I) is...Ch. 2.6 - Which is a stronger base? a. H2O or HO b. H2O or...Ch. 2.7 - Which is a stronger acid? a. CH3OCH2CH2OH or...Ch. 2.7 - Which is a stronger base?Ch. 2.8 - Fosamax has six acidic groups. The structure of...Ch. 2.8 - Which is a stronger acid? Why?Ch. 2.10 - For each of the following compounds (shown in...Ch. 2.10 - Prob. 33PCh. 2.11 - Write the equation that shows how a buffer made by...Ch. 2.11 - What products are formed when each of the...Ch. 2 - a. List the following alcohols in order from...Ch. 2 - Which is a stronger base? a. HS or HO b. CH3O or...Ch. 2 - Prob. 40PCh. 2 - a. List the following carboxylic acids in order...Ch. 2 - For the following compound, a. draw its conjugate...Ch. 2 - List the following compounds in order from...Ch. 2 - For each of the following compounds, draw the form...Ch. 2 - Give the products of the following acidbase...Ch. 2 - Prob. 46PCh. 2 - For each compound, indicate the atom that is most...Ch. 2 - Tenormin, a member of the group of drugs known as...Ch. 2 - From which acids can HO remove a proton in a...Ch. 2 - Prob. 50PCh. 2 - Which is a stronger acid? a. CH29CHCOOH or...Ch. 2 - Prob. 52PCh. 2 - Prob. 53PCh. 2 - How could you separate a mixture of the following...Ch. 2 - Prob. 1PCh. 2 - Prob. 2PCh. 2 - Draw the products of the following acidbase...Ch. 2 - Prob. 4PCh. 2 - Prob. 5PCh. 2 - Prob. 6PCh. 2 - Prob. 7PCh. 2 - Prob. 8PCh. 2 - Prob. 9PCh. 2 - Prob. 10PCh. 2 - Prob. 11PCh. 2 - Prob. 12PCh. 2 - Prob. 13PCh. 2 - Prob. 14P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the calculate the reaction quotient for the following system, if the partial pressure of all reactantsand products is 0.15 atm: NOCl (g) ⇌ NO (g) + Cl2 (g) H = 20.5 kcalarrow_forwardComplete the spectroscopy with structurearrow_forwardcould you answer the questions and draw the complete mechanismarrow_forward
- Complete the spectroscopy with structurearrow_forwardCalculate the reaction quotient for the reaction:NaOH (s) ⇌ Na+ (aq)+ OH- (aq) + 44.4 kJ [Na+] = 4.22 M [OH-] = 6.41 Marrow_forwardGiven the following concentrations for a system, calculate the value for the reaction quotient: Cl2(g)+ CS2(g) ⇌ CCl4(g)+ S2Cl2(g) Cl2 = 31.1 atm CS2 = 91.2 atm CCl4 = 2.12 atm S2Cl2 = 10.4 atmarrow_forward
- Match each chemical or item with the proper disposal or cleanup mwthod, Not all disposal and cleanup methods will be labeled. Metal sheets C, calcium, choroide solutions part A, damp metal pieces Part B, volumetric flask part A. a.Return to correct lables”drying out breaker. Place used items in the drawer.: Rinse with deionized water, dry as best you can, return to instructor. Return used material to the instructor.: Pour down the sink with planty of running water.: f.Pour into aqueous waste container. g.Places used items in garbage.arrow_forwardWrite the equilibrium constant expression for the following reaction: HNO2(aq) + H2O(l) ⇌ H3O+(aq) + NO2-(aq)arrow_forwardWrite the reaction quotient for: Pb2+(aq) + 2 Cl- (aq) ⇌ PbCl2(s)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY