
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 2, Problem 2.59PAE
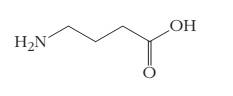
2.59 The accompanying figure shows the structure of gamma-aminoburanoic acid, or GABA. This molecule is a neurotransmitter. Some of the effects of alcohol consumption are due to the interaction between ethanol and GABA. Write the correct molecular formula for this compound.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Consider the structure of 1-bromo-2-fluoroethane.
Part 1 of 2
Draw the Newman projection for the anti conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond.
✡
ぬ
Part 2 of 2
H
H
F
Br
H
H
☑
Draw the Newman projection for the gauche conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond.
H
F
Br
H
H
Please help me answer this question. I don't understand how or where the different reagents will attach and it's mostly due to the wedge bond because I haven't seen a problem like this before. Please provide a detailed explanation and a drawing showing how it can happen and what the final product will look like.
Which of the following compounds is the most acidic in the gas phase?
Group of answer choices
H2O
SiH4
HBr
H2S
Chapter 2 Solutions
Chemistry for Engineering Students
Ch. 2 - Name at least three common polymers and give...Ch. 2 - Prob. 2COCh. 2 - Describe the nuclear model for the atom and...Ch. 2 - Prob. 4COCh. 2 - Prob. 5COCh. 2 - Prob. 6COCh. 2 - Prob. 7COCh. 2 - Prob. 8COCh. 2 - Prob. 9COCh. 2 - Prob. 10CO
Ch. 2 - Prob. 2.1PAECh. 2 - How do polymers compare to their respective...Ch. 2 - Look around you and identify several objects that...Ch. 2 - Prob. 2.4PAECh. 2 - The fact that a polymer’s physical properties...Ch. 2 - One application of conductive polymers is in...Ch. 2 - Prob. 2.7PAECh. 2 - Prob. 2.8PAECh. 2 - Why is the number of protons called the atomic...Ch. 2 - 2.10 Which isotope in each pair contains more...Ch. 2 - 2.11 Define the term isotope.Ch. 2 - 2.12 Write the complete atomic symbol for each of...Ch. 2 - 2.13 How many electrons, protons, and neutrons are...Ch. 2 - 2.14 Consider the following nuclear symbols. How...Ch. 2 - 2.15 Mercury is 16.716 times more massive than...Ch. 2 - The element gallium, used in gallium arsenide...Ch. 2 - 2.17 The atomic weight of copper is 63.55 amu....Ch. 2 - The following table presents the abundances and...Ch. 2 - 2.19 Naturally occurring uranium consists of two...Ch. 2 - Prob. 2.20PAECh. 2 - Prob. 2.21PAECh. 2 - 2.22 Provide the symbol of the following...Ch. 2 - Prob. 2.23PAECh. 2 - 2.24 Identify each of the following species as an...Ch. 2 - 2.25 Write the atomic symbol for the element whose...Ch. 2 - 2.26 In what region of the periodic table are you...Ch. 2 - Prob. 2.27PAECh. 2 - Prob. 2.28PAECh. 2 - Prob. 2.29PAECh. 2 - 2.30 Using Coulomb’s law, explain how the...Ch. 2 - Prob. 2.31PAECh. 2 - 2.32 Which of the following formulas contains the...Ch. 2 - Prob. 2.33PAECh. 2 - Prob. 2.34PAECh. 2 - Prob. 2.35PAECh. 2 - 2.36 Explain the difference between a molecular...Ch. 2 - 2.37 Why are empirical formulas preferred for...Ch. 2 - 2.38 The molecular formula for the ethylene...Ch. 2 - 239 Polybutadiene is a synthetic elastomer, or...Ch. 2 - 2.40 What distinguished the work of Mendeleev that...Ch. 2 - 2.41 How does the periodic table help to make the...Ch. 2 - 2.42 What is a period in the periodic table? From...Ch. 2 - 2.43 Name of the group to which each of the...Ch. 2 - Prob. 2.44PAECh. 2 - Prob. 2.45PAECh. 2 - 2.46 Why are nonmetals important even though they...Ch. 2 - Prob. 2.47PAECh. 2 - A materials engineer has filed for a patent for a...Ch. 2 - Prob. 2.49PAECh. 2 - 2.50 A materials engineer wants to make a new...Ch. 2 - Prob. 2.51PAECh. 2 - Prob. 2.52PAECh. 2 - 2.53 What is meant by the phrase organic...Ch. 2 - 2.54 Based on what you have learned in this...Ch. 2 - 2.55 What is a functional group? How does the...Ch. 2 - Prob. 2.56PAECh. 2 - Prob. 2.57PAECh. 2 - Prob. 2.58PAECh. 2 - 2.59 The accompanying figure shows the structure...Ch. 2 - Prob. 2.60PAECh. 2 - 2.61 Name the following covalent compounds: (a)...Ch. 2 - Prob. 2.62PAECh. 2 - Prob. 2.63PAECh. 2 - Prob. 2.64PAECh. 2 - Prob. 2.65PAECh. 2 - Prob. 2.66PAECh. 2 - Prob. 2.67PAECh. 2 - 2.68 What is a free radical? How are free radicals...Ch. 2 - Prob. 2.69PAECh. 2 - 2.70 Why do you think an inhibitor molecule is...Ch. 2 - 2.71 Use the web to determine the amount of...Ch. 2 - 2.72 How can an element have an atomic weight that...Ch. 2 - 2.73 Explain the concept of a “weighted” average...Ch. 2 - 2.74 The accompanying table provides the identity...Ch. 2 - 2.75 Chlorine has only two isotopes, one with mass...Ch. 2 - Prob. 2.76PAECh. 2 - Prob. 2.77PAECh. 2 - Prob. 2.78PAECh. 2 - Prob. 2.79PAECh. 2 - 2.80 Of the following elements, which two would...Ch. 2 - 2.81 How do binary compounds with hydrogen...Ch. 2 - Prob. 2.82PAECh. 2 - Prob. 2.83PAECh. 2 - 2.84 Early attempts to arrange the elements often...Ch. 2 - 2.85 Describe how the saying “opposites attract”...Ch. 2 - 2.86 For some uses, the relative abundance of...Ch. 2 - 2.87 What is the heaviest element to have an...Ch. 2 - 2.88 Describe how you can identify the isotope, X,...Ch. 2 - Prob. 2.89PAECh. 2 - 2.90 Naturally occurring europium has an average...Ch. 2 - 2.91 Strontium has four stable isotopes....Ch. 2 - 2.92 A candy manufacturer makes chocolate-covered...Ch. 2 - Prob. 2.93PAECh. 2 - 2.94 Use a molecular level description to...Ch. 2 - 2.95 Engineers who design bicycle frames are...Ch. 2 - 2.96 Use the web to look up the density of...Ch. 2 - 2.97 LDPE has a density in the range of...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following is the most acidic transition metal cation? Group of answer choices Fe3+ Sc3+ Mn4+ Zn2+arrow_forwardBased on the thermodynamics of acetic acid dissociation discussed in Lecture 2-5, what can you conclude about the standard enthalpy change (ΔHo) of acid dissociation for HCl? Group of answer choices You cannot arrive at any of the other three conclusions It is a positive value It is more negative than −0.4 kJ/mol It equals −0.4 kJ/molarrow_forwardPLEASE HELP URGENT!arrow_forward
- Draw the skeletal structure corresponding to the following IUPAC name: 7-isopropyl-3-methyldecanearrow_forwardWhich of the following oxyacids is the weakest? Group of answer choices H2SeO3 Si(OH)4 H2SO4 H3PO4arrow_forwardAdd conditions above and below the arrow that turn the reactant below into the product below in a single transformation. + More... If you need to write reagents above and below the arrow that have complex hydrocarbon groups in them, there is a set of standard abbreviations you can use. More... T H,N NC Datarrow_forward
- Indicate the order of basicity of primary, secondary and tertiary amines.arrow_forward> Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic. Cl Z- N O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic O aromatic ○ antiaromatic nonaromaticarrow_forwardPlease help me answer this question. I don't understand how or even if this can happen in a single transformation. Please provide a detailed explanation and a drawing showing how it can happen in a single transformation. Add the necessary reagents and reaction conditions above and below the arrow in this organic reaction. If the products can't be made from the reactant with a single transformation, check the box under the drawing area instead.arrow_forward
- 2) Draw the correct chemical structure (using line-angle drawings / "line structures") from their given IUPAC name: a. (E)-1-chloro-3,4,5-trimethylhex-2-ene b. (Z)-4,5,7-trimethyloct-4-en-2-ol C. (2E,6Z)-4-methylocta-2,6-dienearrow_forwardපිපිම Draw curved arrows to represent the flow of electrons in the reaction on the left Label the reactants on the left as either "Acid" or "Base" (iii) Decide which direction the equilibrium arrows will point in each reaction, based on the given pk, values (a) + H-O H 3-H + (c) H" H + H****H 000 44-00 NH₂ (e) i Дон OH Ө NHarrow_forward3) Label the configuration in each of the following alkenes as E, Z, or N/A (for non-stereogenic centers). 00 E 000 N/A E Br N/A N/A (g) E N/A OH E (b) Oz N/A Br (d) 00 E Z N/A E (f) Oz N/A E (h) Z N/Aarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
GCSE Chemistry - Differences Between Compounds, Molecules & Mixtures #3; Author: Cognito;https://www.youtube.com/watch?v=jBDr0mHyc5M;License: Standard YouTube License, CC-BY