
Concept explainers
Fill in the names beside the symbols of the following elements commonly found in living matter.
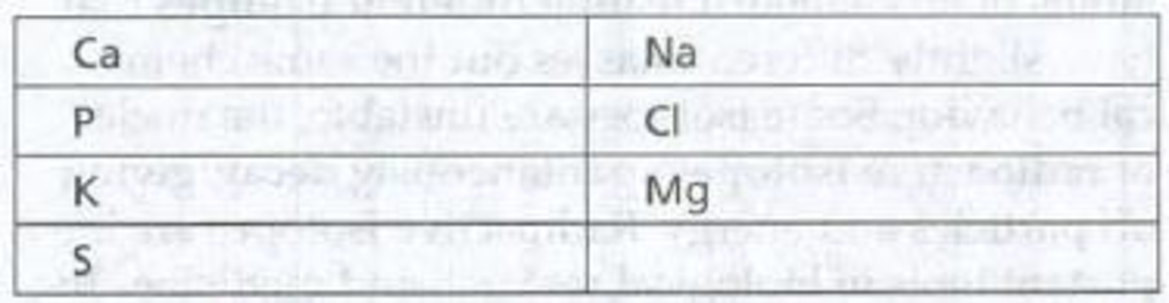
To fill: The names beside the symbol of the given element that are commonly found in living matter.
Introduction: In the simplest level of organization, the human body consists of various chemical structures. The substances that cannot be broken down into simpler substances by ordinary chemical reactions are called elements. Every element has its own chemical symbol.
Answer to Problem 1IQ
Tabular representation: The names of the symbol of the given element are in Table 1.
Table 1: Names of the symbol of the given element
| Ca -Calcium | Na- Sodium |
| P- Phosphorus | Cl- Chlorine |
| K- Potassium | Mg- Magnesium |
| S- Sulfur |
Explanation of Solution
Chemical elements are represented as symbols. In general, first one or two letters of element’s name are represented as a symbol of an element. For example, sulfur is represented as symbol “S” and calcium is represented as “Ca”. But few elements have symbols that have no relationship with their names. For example, potassium is represented by symbol “K” and sodium is represented as “Na”. For such cases, origin of the elements is used.
- Potassium- K- Latin name Kalium –means alkali
- Sodium- Na- Latin name for sodium carbonate- Natrium
Want to see more full solutions like this?
Chapter 2 Solutions
Study Guide for Campbell Biology
- You implant an FGF10-coated bead into the anterior flank of a chicken embryo, directly below the level of the wing bud. What is the phenotype of the resulting ectopic limb? Briefly describe the expected expression domains of 1) Shh, 2) Tbx4, and 3) Tbx5 in the resulting ectopic limb bud.arrow_forwardDesign a grafting experiment to determine if limb mesoderm determines forelimb / hindlimb identity. Include the experiment, a control, and an interpretation in your answer.arrow_forwardThe Snapdragon is a popular garden flower that comes in a variety of colours, including red, yellow, and orange. The genotypes and associated phenotypes for some of these flowers are as follows: aabb: yellow AABB, AABb, AaBb, and AaBB: red AAbb and Aabb: orange aaBB: yellow aaBb: ? Based on this information, what would the phenotype of a Snapdragon with the genotype aaBb be and why? Question 21 options: orange because A is epistatic to B yellow because A is epistatic to B red because B is epistatic to A orange because B is epistatic to A red because A is epistatic to B yellow because B is epistatic to Aarrow_forward
- A sample of blood was taken from the above individual and prepared for haemoglobin analysis. However, when water was added the cells did not lyse and looked normal in size and shape. The technician suspected that they had may have made an error in the protocol – what is the most likely explanation? The cell membranes are more resistant than normal. An isotonic solution had been added instead of water. A solution of 0.1 M NaCl had been added instead of water. Not enough water had been added to the red blood cell pellet. The man had sickle-cell anaemia.arrow_forwardA sample of blood was taken from the above individual and prepared for haemoglobin analysis. However, when water was added the cells did not lyse and looked normal in size and shape. The technician suspected that they had may have made an error in the protocol – what is the most likely explanation? The cell membranes are more resistant than normal. An isotonic solution had been added instead of water. A solution of 0.1 M NaCl had been added instead of water. Not enough water had been added to the red blood cell pellet. The man had sickle-cell anaemia.arrow_forwardWith reference to their absorption spectra of the oxy haemoglobin intact line) and deoxyhemoglobin (broken line) shown in Figure 2 below, how would you best explain the reason why there are differences in the major peaks of the spectra? Figure 2. SPECTRA OF OXYGENATED AND DEOXYGENATED HAEMOGLOBIN OBTAINED WITH THE RECORDING SPECTROPHOTOMETER 1.4 Abs < 0.8 06 0.4 400 420 440 460 480 500 520 540 560 580 600 nm 1. The difference in the spectra is due to a pH change in the deoxy-haemoglobin due to uptake of CO2- 2. There is more oxygen-carrying plasma in the oxy-haemoglobin sample. 3. The change in Mr due to oxygen binding causes the oxy haemoglobin to have a higher absorbance peak. 4. Oxy-haemoglobin is contaminated by carbaminohemoglobin, and therefore has a higher absorbance peak 5. Oxy-haemoglobin absorbs more light of blue wavelengths and less of red wavelengths than deoxy-haemoglobinarrow_forward
- With reference to their absorption spectra of the oxy haemoglobin intact line) and deoxyhemoglobin (broken line) shown in Figure 2 below, how would you best explain the reason why there are differences in the major peaks of the spectra? Figure 2. SPECTRA OF OXYGENATED AND DEOXYGENATED HAEMOGLOBIN OBTAINED WITH THE RECORDING SPECTROPHOTOMETER 1.4 Abs < 0.8 06 0.4 400 420 440 460 480 500 520 540 560 580 600 nm 1. The difference in the spectra is due to a pH change in the deoxy-haemoglobin due to uptake of CO2- 2. There is more oxygen-carrying plasma in the oxy-haemoglobin sample. 3. The change in Mr due to oxygen binding causes the oxy haemoglobin to have a higher absorbance peak. 4. Oxy-haemoglobin is contaminated by carbaminohemoglobin, and therefore has a higher absorbance peak 5. Oxy-haemoglobin absorbs more light of blue wavelengths and less of red wavelengths than deoxy-haemoglobinarrow_forwardWhich ONE of the following is FALSE regarding haemoglobin? It has two alpha subunits and two beta subunits. The subunits are joined by disulphide bonds. Each subunit covalently binds a haem group. Conformational change in one subunit can be transmitted to another. There are many variant ("mutant") forms of haemoglobin that are not harmful.arrow_forwardWhich ONE of the following is FALSE regarding haemoglobin? It has two alpha subunits and two beta subunits. The subunits are joined by disulphide bonds. Each subunit covalently binds a haem group. Conformational change in one subunit can be transmitted to another. There are many variant ("mutant") forms of haemoglobin that are not harmful.arrow_forward
- During a routine medical check up of a healthy man it was found that his haematocrit value was highly unusual – value of 60%. What one of the options below is the most likely reason? He will have a diet high in iron. He is likely to be suffering from anaemia. He lives at high altitude. He has recently recovered from an accident where he lost a lot of blood. He has a very large body size.arrow_forwardExplain what age of culture is most likely to produce an endospore?arrow_forwardExplain why hot temperatures greater than 45 degrees celsius would not initiate the sporulation process in endospores?arrow_forward
 Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning





