
EBK ORGANIC CHEMISTRY
9th Edition
ISBN: 9780100591318
Author: McMurry
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19.SE, Problem 46MP
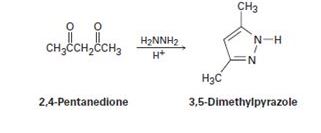
Propose a mechanism to account for the formation of 3, 5-dimethyl- pyrazole from hydrazine and 2, 4-pentanedione. Look carefully to see what has happened to each carbonyl carbon in going from starting material to product.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
What is the final product when D-galactose reacts with hydroxylamine?
Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.
Chapter 19 Solutions
EBK ORGANIC CHEMISTRY
Ch. 19.1 - Prob. 1PCh. 19.1 - Draw structures corresponding to the following...Ch. 19.2 - Prob. 3PCh. 19.2 - How would you carry out the following reactions?...Ch. 19.4 - Treatment of an aldehyde or ketone with cyanide...Ch. 19.4 - p-Nitrobenzaldehyde is more reactive toward...Ch. 19.5 - Prob. 7PCh. 19.5 - The oxygen in water is primarily (99.8) 16O, but...Ch. 19.6 - Prob. 9PCh. 19.8 - Show the products you would obtain by...
Ch. 19.8 - Prob. 11PCh. 19.8 - Prob. 12PCh. 19.9 - Prob. 13PCh. 19.10 - Prob. 14PCh. 19.10 - Prob. 15PCh. 19.11 - What carbonyl compound and what phosphorus ylide...Ch. 19.11 - -Carotene, a yellow food-coloring agent and...Ch. 19.12 - Prob. 18PCh. 19.12 - Prob. 19PCh. 19.13 - Prob. 20PCh. 19.13 - Treatment of 2-cyclohexenone with HCN/KCN yields a...Ch. 19.13 - How might conjugate addition reactions of lithium...Ch. 19.14 - How might you use IR spectroscopy to determine...Ch. 19.14 - Prob. 24PCh. 19.14 - Prob. 25PCh. 19.14 - Prob. 26PCh. 19.SE - Each of the following substances can be prepared...Ch. 19.SE - Prob. 28VCCh. 19.SE - Prob. 29VCCh. 19.SE - Prob. 30MPCh. 19.SE - Prob. 31MPCh. 19.SE - Prob. 32MPCh. 19.SE - Prob. 33MPCh. 19.SE - Prob. 34MPCh. 19.SE - Prob. 35MPCh. 19.SE - It is not uncommon for organic chemists to prepare...Ch. 19.SE - Prob. 37MPCh. 19.SE - Prob. 38MPCh. 19.SE - Prob. 39MPCh. 19.SE - Prob. 40MPCh. 19.SE - Aldehydes and ketones react with thiols to yield...Ch. 19.SE - Prob. 42MPCh. 19.SE - When cyclohexanone is heated in the presence of a...Ch. 19.SE - Prob. 44MPCh. 19.SE - The Meerwein-Ponndorf-Verley reaction involves...Ch. 19.SE - Propose a mechanism to account for the formation...Ch. 19.SE - Prob. 47MPCh. 19.SE - Prob. 48MPCh. 19.SE - Treatment of an , -unsaturated ketone with basic...Ch. 19.SE - Prob. 50MPCh. 19.SE - Prob. 51MPCh. 19.SE - Prob. 52MPCh. 19.SE - Prob. 53MPCh. 19.SE - Prob. 54APCh. 19.SE - Draw and name the seven aldehydes and ketones with...Ch. 19.SE - Give IUPAC names for the following compounds:Ch. 19.SE - Draw structures of compounds that fit the...Ch. 19.SE - Predict the products of the reaction of (1)...Ch. 19.SE - Show how you might use a Wittig reaction to...Ch. 19.SE - How would you use a Grignard reaction on an...Ch. 19.SE - Prob. 61APCh. 19.SE - Prob. 62APCh. 19.SE - How would you synthesize the following substances...Ch. 19.SE - Carvone is the major constituent of spearmint oil....Ch. 19.SE - How would you synthesize the following compounds...Ch. 19.SE - At what position would you expect to observe IR...Ch. 19.SE - Acidcatalyzed dehydration of...Ch. 19.SE - Choose the structure that best fits the IR...Ch. 19.SE - Propose structures for molecules that meet the...Ch. 19.SE - Prob. 70APCh. 19.SE - Prob. 71APCh. 19.SE - When 4hydroxybutanal is treated with methanol in...Ch. 19.SE - Prob. 73APCh. 19.SE - Prob. 74APCh. 19.SE - Prob. 75APCh. 19.SE - Prob. 76APCh. 19.SE - Prob. 77APCh. 19.SE - Tamoxifen is a drug used in the treatment of...Ch. 19.SE - Compound A, MW 86, shows an IR absorption at 1730...Ch. 19.SE - Compound B is isomeric with A (Problem 19-79) and...Ch. 19.SE - The 1HNMR spectrum shown is that of a compound...Ch. 19.SE - Prob. 82APCh. 19.SE - Propose structures for ketones or aldehydes that...Ch. 19.SE - Prob. 84APCh. 19.SE - Prob. 85APCh. 19.SE - The proton and carbon NMR spectra for each of...Ch. 19.SE - The proton NMR spectrum for a compound with...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License