
Concept explainers
(a)
Interpretation:
Systematic name of the given compound has to be given.
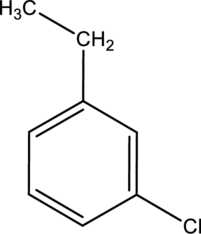
Concept introduction:
IUAC gives rules for the naming of chemical compounds. These rules are,
For disubstituted benzene compounds, take benzene with one substituent as a parent compound.
Add a prefix with another substituent like ortho ortho-, meta- and para- followed by parent name.
o-ortho for a substituent, which is present at adjacent to the parent substituent.
m-meta for a substituent, which is present at one carbon apart to the parent substituent.
p-para for a substituent, which is present at opposite to the parent substituent.
(b)
Interpretation:
Systematic name of the given compound has to be given.
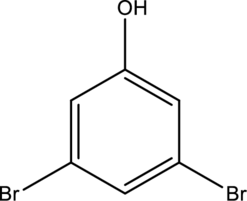
Concept introduction:
IUPAC Nomenclature:
IUAC gives rules for the naming of chemical compounds. These rules are,
For disubstituted benzene compounds, take benzene as a parent compound.
Number the benzene ring by assigning the lowest priorities to the substituents.
Write the position number and substituent name by following alphabet letters then write the parent name of the compound.
If more than one same substitutions are present in a compound means add a prefix like di, tri, tetra, ect…
(c)
Interpretation:
Systematic name of the given compound has to be given.
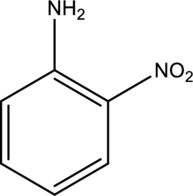
Concept introduction:
Refer part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
Foundations of College Chemistry, Binder Ready Version
- Steps and explanation please. Add how to solve or target similar problems.arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forwardThis organic molecule is dissolved in an acidic aqueous solution: OH OH A short time later sensitive infrared spectroscopy reveals the presence of a new C = O stretch absorption. That is, there must now be a new molecule present with at least one C = O bond. In the drawing area below, show the detailed mechanism that could convert the molecule above into the new molecule. Videos 849 Explanation Check C Click and drag to start dwing a structure. # 3 MAR 23 Add/Remove steparrow_forward||| 7:47 ull 57% ← Problem 19 of 48 Submit Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the product of this carbocation rearrangement. Include all lone pairs and charges as appropriate. H 1,2-alkyl shift +arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forwardBelow is the SN1 reaction of (S)-3-chlorocyclohexene and hydroxide (OH). Draw the missing curved arrows, lone pairs of electrons, and nonzero formal charges. In the third box, draw the two enantiomeric products that will be produced. 5th attempt Please draw all four bonds at chiral centers. Draw the two enantiomeric products that will be produced. Draw in any hydrogen at chiral centers. 1000 4th attempt Feedback Please draw all four bonds at chiral centers. 8. R5 HO: See Periodic Table See Hint H Cl Br Jid See Periodic Table See Hintarrow_forwardShow that a molecule with configuration π4 has a cylindrically symmetric electron distribution. Hint: Let the π orbitals be equal to xf and yf, where f is a function that depends only on the distance from the internuclear axis.arrow_forward(a) Verify that the lattice energies of the alkali metal iodides are inversely proportional to the distances between the ions in MI (M = alkali metal) by plotting the lattice energies given below against the internuclear distances dMI. Is the correlation good? Would a better fit be obtained by plotting the lattice energies as a function of (1 — d*/d)/d, as theoretically suggested, with d* = 34.5 pm? You must use a standard graphing program to plot the graph. It generates an equation for the line and calculates a correlation coefficient. (b) From the graph obtained in (a), estimate the lattice energy of silver iodide. (c) Compare the results of (b) with the experimental value of 886 kJ/mol. If they do not agree, explain the deviation.arrow_forwardCan I please get help with #3 & 4? Thanks you so much!arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning


