
Concept explainers
Consider an
- (a) Diagram the cell, and label each of the components (including the anode, cathode, and salt bridge).
- (b) Use the equations for the half-reactions to write a balanced, net ionic equation for the overall cell reaction.
- (c) What is the polarity of each electrode?
- (d) What is the value of E°cell?
- (e) In which direction do electrons flow in the external circuit?
- (f) Assume that a salt bridge containing NaNO3 connects the two half-cells. In which direction do the Na+(aq) ions move? In which direction do the NO3− (aq) ions move?
- (g) Calculate the equilibrium constant for the reaction.
- (h) If the concentration of Cd2+ is reduced to 0.010 M and [Ni2+] = 1.0 M, what is the value of Ecell? Is the net reaction still the reaction given in part (b)?
- (i) If 0.050 A is drawn from the battery, how long can it last if you begin with 1.0 L of each of the solutions and each was initially 1.0 M in dissolved species? Each electrode weighs 50.0 g in the beginning.
(a)
Interpretation:
The half reactions are as follows.
The cell has to be drawn and label each of the component.
Concept introduction:
Voltaic cell or Galvanic cell:
The device to produce electricity by using chemical reactions. In these divces are redox chemical reactions are occured.
A voltaic cell converts chemical energy into electrical energy.
It consists of two half cells. Each half cell consists of a metal and a solution of a salt of metal. Two half cells are connected by salt bridge.
The chemical reaction in the half cell is an oxidation reduction (redox)reactions.
For example:
Cell diagram of voltaic or galvanic cell is as follows.
Answer to Problem 99IL
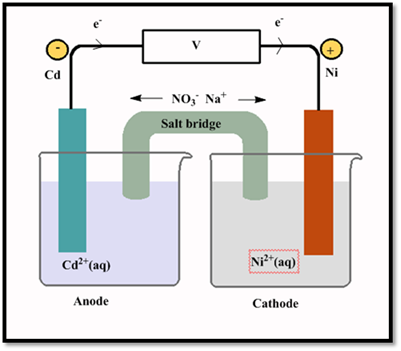
Explanation of Solution
The half reactions are as follows.
The voltaic cell are as follows:
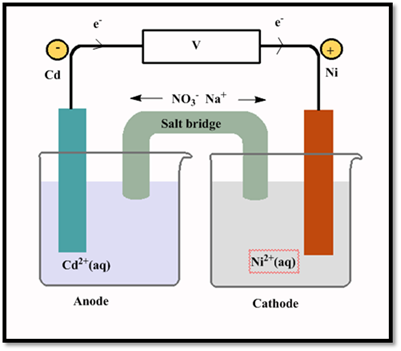
(b)
Interpretation:
To determine the following.
The half reactions are as follows.
The balance equation has to be given.
Concept introduction:
Voltaic cell or Galvanic cell:
The device to produce electricity by using chemical reactions. In these divces are redox chemical reactions are occured.
A voltaic cell converts chemical energy into electrical energy.
It consists of two half cells. Each half cell consists of a metal and a solution of a salt of metal. Two half cells are connected by salt bridge.
The chemical reaction in the half cell is an oxidation reduction (redox)reactions.
For example:
Cell diagram of voltaic or galvanic cell is as follows.
Answer to Problem 99IL
Balanced reaction:
Explanation of Solution
Let’s write the half reactions occur at anode and cathode:
By adding these two half reactions we get balanced reaction.
(c)
Interpretation:
The half reactions are as follows.
The polarity of each electrode has to be determined.
Concept introduction:
Voltaic cell or Galvanic cell:
The device to produce electricity by using chemical reactions. In these divces are redox chemical reactions are occured.
A voltaic cell converts chemical energy into electrical energy.
It consists of two half cells. Each half cell consists of a metal and a solution of a salt of metal. Two half cells are connected by salt bridge.
The chemical reaction in the half cell is an oxidation reduction (redox)reactions.
For example:
Cell diagram of voltaic or galvanic cell is as follows.
Answer to Problem 99IL
Anode is negative cathode is positive.
Explanation of Solution
In the voltaic cell has two voltaic cells. One electrode has positive charge called cathode and another electrode has negative called anode.
(d)
Interpretation:
The half reactions are as follows.
The
Concept introduction:
Voltaic cell or Galvanic cell:
The device to produce electricity by using chemical reactions. In these divces are redox chemical reactions are occured.
A voltaic cell converts chemical energy into electrical energy.
It consists of two half cells. Each half cell consists of a metal and a solution of a salt of metal. Two half cells are connected by salt bridge.
The chemical reaction in the half cell is an oxidation reduction (redox)reactions.
For example:
Cell diagram of voltaic or galvanic cell is as follows.
Answer to Problem 99IL
Explanation of Solution
The reactions occur at anode and cathode is as follows.
Let’s calculate the
(e)
Interpretation:
The half reactions are as follows.
The direction in which electrons flow in the external circuit has to be given.
Concept introduction:
Voltaic cell or Galvanic cell:
The device to produce electricity by using chemical reactions. In these divces are redox chemical reactions are occured.
A voltaic cell converts chemical energy into electrical energy.
It consists of two half cells. Each half cell consists of a metal and a solution of a salt of metal. Two half cells are connected by salt bridge.
The chemical reaction in the half cell is an oxidation reduction (redox)reactions.
For example:
Cell diagram of voltaic or galvanic cell is as follows.
Answer to Problem 99IL
Electrons are flow from anode to cathode.
Explanation of Solution
In the voltaic cell electrons are move anode to cathode.
(f)
Interpretation:
To determine the following.
The half reactions are as follows.
Assume that a salt bride containing
Concept introduction:
Voltaic cell or Galvanic cell:
The device to produce electricity by using chemical reactions. In these divces are redox chemical reactions are occured.
A voltaic cell converts chemical energy into electrical energy.
It consists of two half cells. Each half cell consists of a metal and a solution of a salt of metal. Two half cells are connected by salt bridge.
The chemical reaction in the half cell is an oxidation reduction (redox)reactions.
For example:
Cell diagram of voltaic or galvanic cell is as follows.
Answer to Problem 99IL
Explanation of Solution
Salt bridge contains
(g)
Interpretation:
The half reactions are as follows.
The equilibrium constant has to be determined.
Concept introduction:
Voltaic cell or Galvanic cell:
The device to produce electricity by using chemical reactions. In these divces are redox chemical reactions are occured.
A voltaic cell converts chemical energy into electrical energy.
It consists of two half cells. Each half cell consists of a metal and a solution of a salt of metal. Two half cells are connected by salt bridge.
The chemical reaction in the half cell is an oxidation reduction (redox)reactions.
For example:
Cell diagram of voltaic or galvanic cell is as follows.
Answer to Problem 99IL
The equilibrium constant of the reaction is
Explanation of Solution
(h)
Interpretation:
The half reactions are as follows.
If the concentration of
Concept introduction:
Voltaic cell or Galvanic cell:
The device to produce electricity by using chemical reactions. In these divces are redox chemical reactions are occured.
A voltaic cell converts chemical energy into electrical energy.
It consists of two half cells. Each half cell consists of a metal and a solution of a salt of metal. Two half cells are connected by salt bridge.
The chemical reaction in the half cell is an oxidation reduction (redox)reactions.
For example:
Cell diagram of voltaic or galvanic cell is as follows.
Answer to Problem 99IL
Explanation of Solution
Net reaction will be still given in part (b)
(i)
Interpretation:
The half reactions are as follows.
The time the battery will last if
Concept introduction:
Voltaic cell or Galvanic cell:
The device to produce electricity by using chemical reactions. In these divces are redox chemical reactions are occured.
A voltaic cell converts chemical energy into electrical energy.
It consists of two half cells. Each half cell consists of a metal and a solution of a salt of metal. Two half cells are connected by salt bridge.
The chemical reaction in the half cell is an oxidation reduction (redox)reactions.
For example:
Cell diagram of voltaic or galvanic cell is as follows.
Answer to Problem 99IL
The time required for the electrolysis is
Explanation of Solution
Let’s calculate the charge of the cell:
Therefore, the time can be calculated as follows.
Want to see more full solutions like this?
Chapter 19 Solutions
Chemistry & Chemical Reactivity
- QUESTION: Find the standard deviation for the 4 different groups 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5.033 4.044 334.6 268.7 4.706 3.621 305.6 234.4 4.816 3.728 340.0 262.7 4.828 4.496 304.3 283.2 4.993 3.865 244.7 143.6 STDEV = STDEV = STDEV = STDEV =arrow_forwardQUESTION: Fill in the answers in the empty green boxes regarding 'Question 5: Calculating standard error of regression' *The images of the data showing 'coefficients for the standard curve' have been providedarrow_forwardUsing the Nernst equation to calculate nonstandard cell voltage Try Again Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 2+ 2+ Sn²+ Ba(s) (aq) + Ba (s) Sn (s) + Ba²+ (aq) →>> Suppose the cell is prepared with 6.10 M Sn 2+ 2+ in one half-cell and 6.62 M Ba in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. 1.71 V ☐ x10 ☑ 5 0/5 ? 00. 18 Ararrow_forward
- Question: Find both the b (gradient) and a (y-intercept) value from the list of data below: (x1 -x̄) 370.5 (y1 - ȳ) 5.240 (x2 - x̄) 142.5 (y2 - ȳ) 2.004 (x3 - x̄) 28.5 (y3 - ȳ) 0.390 (x4 - x̄) -85.5 (y4 - ȳ) -1.231 (x5 - x̄) -199.5 (y5 - ȳ) -2.829 (x6 - x̄) -256.5 (y6 - ȳ) -3.575arrow_forwardCalculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3Cu+ (aq) + Cro²¯ (aq) +4H₂O (1) → 3Cu²+ (aq) +Cr(OH)3 (s)+5OH˜¯ (aq) 0 kJ ☐ x10 00. 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 241.7 mL of a 0.4900M solution of methylamine (CH3NH2) with a 0.7800M solution of HNO3. The pK of methylamine is 3.36. Calculate the pH of the base solution after the chemist has added 17.7 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☑ ? 18 Ararrow_forward
- The following is two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0mmol/L 262.7mmol/L QUESTION: For both groups (Regular & Salt Reduced tomato sauce) of data provide answers to the following calculations below: 1. Standard Deviation (Sx) 2. T Values (t0.05,4) 3. 95% Confidence Interval (mmol/L) 4. [Na+] (mg/100 mL) 5. 95% Confidence Interval (mg/100 mL)arrow_forwardIf we have leucine (2-amino-4-methylpentanoic acid), alanine (2-aminopropanoic acid) and phenylalanine (2-amino-3-phenylpropanoic acid), indicate the tripeptides that can be formed (use the abbreviated symbols Leu., Ala and Phe).arrow_forward
- Briefly state why trifluoroacetic acid is more acidic than acetic acid.arrow_forwardExplain why acid chlorides are more reactive than amides in reactions with nucleophiles.arrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 101.7 mL of a 0.3500M solution of piperidine (C5H10NH) with a 0.05700M solution of HClO4. The pK of piperidine is 2.89. Calculate the pH of the base solution after the chemist has added 682.9 mL of the HClO solution to it. 4 Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO solution added. 4 Round your answer to 2 decimal places. pH = .11 00. 18 Ararrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





