
Fundamentals of Physics Extended
10th Edition
ISBN: 9781118230725
Author: David Halliday, Robert Resnick, Jearl Walker
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 19, Problem 89P
A pipe of length L = 25.0 m that is open at one end contains air at atmospheric pressure. It is thrust vertically into a freshwater lake until the water rises halfway up in the pipe (Fig. 19-30). What is the depth h of the lower end of the pipe? Assume that the temperature is the same everywhere and does not change.
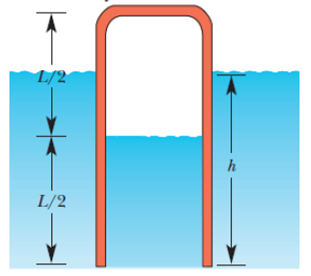
Figure 19-30 Problem 89.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The cylindrical beam of a 12.7-mW laser is 0.920 cm in diameter. What is the rms value of the electric field?
V/m
Consider a rubber rod that has been rubbed with fur to give the rod a net negative charge, and a glass rod that has been rubbed with silk to give it a net positive charge. After being charged by contact by the fur and silk...?
a. Both rods have less mass
b. the rubber rod has more mass and the glass rod has less mass
c. both rods have more mass
d. the masses of both rods are unchanged
e. the rubber rod has less mass and the glass rod has mroe mass
8) 9)
Chapter 19 Solutions
Fundamentals of Physics Extended
Ch. 19 - For four situations for an ideal gas, the table...Ch. 19 - In the p-V diagram of Fig. 19-17, the gas does 5 J...Ch. 19 - For a temperature increase of T1, a certain amount...Ch. 19 - The dot in Fig, 19-18a represents the initial...Ch. 19 - A certain amount of energy is to be transferred as...Ch. 19 - The dot in Fig. 19-18b represents the initial...Ch. 19 - a Rank the four paths of Fig. 19-16 according to...Ch. 19 - The dot in Fig. 19-18c represents the initial...Ch. 19 - Prob. 9QCh. 19 - Does the temperature of an ideal gas increase,...
Ch. 19 - Prob. 1PCh. 19 - Gold has a molar mass of 197 g/mol. a How many...Ch. 19 - SSM Oxygen gas having a volume of 1000 cm3 at...Ch. 19 - A quantity of ideal gas at: 10.0C and 100 kPa...Ch. 19 - The best laboratory vacuum has a pressure of about...Ch. 19 - Water bottle in a hot car. In the American...Ch. 19 - Suppose 1.80 mol of an ideal gas is taken from a...Ch. 19 - Compute a the number of moles and b the number of...Ch. 19 - An automobile tire has a volume of 1.64 102 m3...Ch. 19 - A container encloses 2 mol of an ideal gas that...Ch. 19 - SSM ILW WWW Air that initially occupies 0.140 m3...Ch. 19 - GO Submarine rescue. When the U.S. submarine...Ch. 19 - Prob. 13PCh. 19 - In the temperature range 310 K to 330 K, the...Ch. 19 - Suppose 0.825 mol of an ideal gas undergoes an...Ch. 19 - An air bubble of volume 20 cm3 is at the bottom of...Ch. 19 - GO Container A in Fig. 19-22 holds an ideal gas at...Ch. 19 - The temperature and pressure in the Suns...Ch. 19 - a Compute the rms speed of a nitrogen molecule at...Ch. 19 - Calculate the rms speed of helium atoms at 1000 K....Ch. 19 - SSM The lowest possible temperature in outer space...Ch. 19 - Find the rms speed of argon atoms at 313 K. See...Ch. 19 - A beam of hydrogen molecules H2 is directed toward...Ch. 19 - At 273 K and 1.00 102 atm, the density of a gas...Ch. 19 - Prob. 25PCh. 19 - Prob. 26PCh. 19 - Water standing in the open at 32.0C evaporates...Ch. 19 - At what frequency would the wavelength of sound in...Ch. 19 - SSM The atmospheric density at an altitude of 2500...Ch. 19 - Prob. 30PCh. 19 - In a certain particle accelerator, protons travel...Ch. 19 - Prob. 32PCh. 19 - Prob. 33PCh. 19 - Prob. 34PCh. 19 - Prob. 35PCh. 19 - The most probable speed of the molecules in a gas...Ch. 19 - Prob. 37PCh. 19 - Figure 19-24 gives the probability distribution...Ch. 19 - At what temperature does the rms speed of a...Ch. 19 - Two containers are at the same temperature. The...Ch. 19 - Prob. 41PCh. 19 - What is the internal energy of 1.0 mol of an ideal...Ch. 19 - Prob. 43PCh. 19 - GO One mole of ail ideal diatomic gas goes from a...Ch. 19 - ILW The mass of a gas molecule can be computed...Ch. 19 - Under constant pressure, the temperature of 2.00...Ch. 19 - The temperature of 2.00 mol of an ideal monatomic...Ch. 19 - GO When 20.9 J was added as heat to a particular...Ch. 19 - SSM A container holds a mixture of three...Ch. 19 - We give 70 J as heat to a diatomic gas, which then...Ch. 19 - Prob. 51PCh. 19 - GO Suppose 12.0 g of oxygen O2 gas is heated at...Ch. 19 - SSM WWW Suppose 4.00 mol of an ideal diatomic gas...Ch. 19 - We know that for an adiabatic process pV = a...Ch. 19 - A certain gas occupies a volume of 4.3 L at a...Ch. 19 - Suppose 1.00 L of a gas with = 1.30, initially at...Ch. 19 - The volume of an ideal gas is adiabatically...Ch. 19 - GO Opening champagne. In a bottle of champagne,...Ch. 19 - GO Figure 19-26 shows two paths that may be taken...Ch. 19 - GO Adiabatic wind. The normal airflow over the...Ch. 19 - GO A gas is to be expanded from initial state i to...Ch. 19 - GO An ideal diatomic gas, with rotation but no...Ch. 19 - Figure 19-27 shows a cycle undergone by 1.00 mol...Ch. 19 - Calculate the work done by an external agent...Ch. 19 - An ideal gas undergoes an adiabatic compression...Ch. 19 - Prob. 66PCh. 19 - An ideal monatomic gas initially has a temperature...Ch. 19 - Prob. 68PCh. 19 - SSM The envelope and basket of a hot-air balloon...Ch. 19 - An ideal gas, at initial temperature T1 and...Ch. 19 - Prob. 71PCh. 19 - At what temperature do atoms of helium gas have...Ch. 19 - Prob. 73PCh. 19 - Prob. 74PCh. 19 - The temperature of 3.00 mol of a gas with CV =...Ch. 19 - During a compression at a constant pressure of 250...Ch. 19 - SSM Figure 19-28 shows a hypothetical speed...Ch. 19 - Prob. 78PCh. 19 - SSM An ideal gas undergoes isothermal compression...Ch. 19 - Oxygen O2 gas at 273 K and 1.0 atm is confined to...Ch. 19 - An ideal pas is taken through a complete cycle in...Ch. 19 - Prob. 82PCh. 19 - SSM A sample of ideal gas expands from an initial...Ch. 19 - An ideal gas with 3.00 mol is initially in state 1...Ch. 19 - A steel lank contains 300 g of ammonia gas NH3 at...Ch. 19 - In an industrial process the volume of 25.0 mol of...Ch. 19 - Figure 19-29 shows a cycle consisting of five...Ch. 19 - An ideal gas initially at 300 K is compressed at a...Ch. 19 - A pipe of length L = 25.0 m that is open at one...Ch. 19 - In a motorcycle engine, a piston is forced down...Ch. 19 - For adiabatic processes in an ideal gas, show that...Ch. 19 - Air at 0.000C and 1.00 atm pressure has a density...Ch. 19 - Prob. 93PCh. 19 - Prob. 94PCh. 19 - Prob. 95PCh. 19 - For air near 0C, by how much does the speed of...Ch. 19 - Prob. 97P
Additional Science Textbook Solutions
Find more solutions based on key concepts
27. A dentist uses a curved mirror to view the back side of teeth on the upper jaw. Suppose she wants an erect ...
College Physics: A Strategic Approach (3rd Edition)
Match each of the following items with all the terms it applies to:
Human Physiology: An Integrated Approach (8th Edition)
What is the difference between transcription and translation?
Principles of Anatomy and Physiology
41. A reaction in which A, B, and C react to form products is first order in A, second order in B, and zero ord...
Chemistry: Structure and Properties (2nd Edition)
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
You microscopically examine scrapings from a case of Acan-thamoeba keratitis. You expect to see a. nothing. b. ...
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Lab 8 Part 3 PHET Wave Interface simulation. I am having trouble with this part of the lab.arrow_forwardMick and Rick are twins born on Earth in the year 2175. Rick grows up to be an Earth-bound robotics technician while Mick becomes an intergalactic astronaut. Mick leaves the Earth on his first space mission in the year 2200 and travels, according to his clock, for 10 years at a speed of 0.75c. Unfortunately, at this point in his journey, the structure of his ship undergoes mechanical breakdown and the ship explodes. How old is Rick when his brother dies?arrow_forwardHi, I have canceled, why did you charge me again?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning


Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Thermal Expansion and Contraction of Solids, Liquids and Gases; Author: Knowledge Platform;https://www.youtube.com/watch?v=9UtfegG4DU8;License: Standard YouTube License, CC-BY