
Concept explainers
Interpretation:
The molecular formula of the given compound has to be determined by using the ideal gas equation and molar mass of that compound. The possible structures of the compound has to be drawn from the determined molecular formula.
Concept Introduction:
Ideal gas equation:
The ideal gas equation is the combined equation of
Explanation of Solution
The given information in the problem is recored as follows:
The given temperature is converted to kelvin as shown here.
The given volume in millilitres is converted into liters by the conversion factor as follows:
Use the below ideal gas equation to find the moles of the vapour.
Substitute as follows.
Use the below equation to find the molar mass of the compound.
Substitute as follows.
The empirical formula of the compound can be calculated as follows:
Assume that the mass of the sample is
The grams of each element is converted into moles by using the molar mass of the corresponding element.
Now the number of moles of each element is converted into whole numbers, dividing by the lowest mole.
Thus, the empirical formula of the compound is
The molecular formula of the compound can be determined from the empirical formula and the molar mass of that compound as follows.
Use the below equation to find the molecular formula.
Substitute as follows.
Hence, the molecular formula of the compound is
The determined molecular formula for the compound is
There are two carbon atoms in the given compound. So, place the two carbon atoms in a straight-line as the parent carbon chain as shown below.
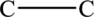
Place the two chlorine atoms on the same carbon atom or for each carbon atom as shown below.
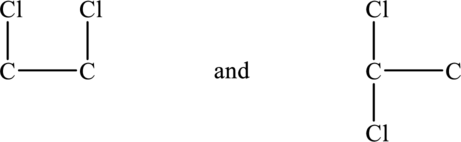
Now add four hydrogen atoms to each carbon atom in order to complete the two structures above because each carbon has four bonds around it. ]
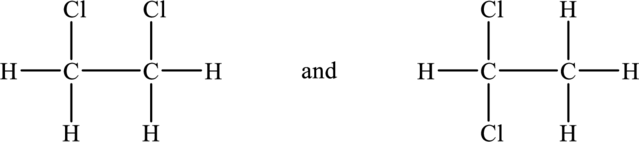
Hence, the possible structures of the compound are show below.
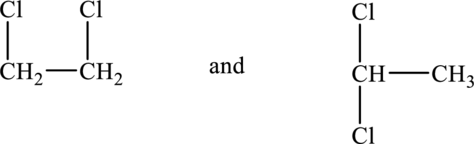
Want to see more full solutions like this?
Chapter 19 Solutions
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
- 22arrow_forwardPLEASE READ!!! I DONT WANT EXAMPLES, I DONT WANT WORDS OR PARAGRAPHS FOR THE MECHANISM!!! THANKS First image: QUESTION 6. I have to show, with ARROWS and STRUCTURES, the mechanism of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary. I also tried to draw the mechanism, tell me what to change. Please note that its an AMIDE thats formed not an AMINE the nitrogen has ONE hydrogen and one Phenyl-C-Phenyl. I already asked for this mechanism and got as a final product ...-NH2 not whats shown on the picture, thank you Ths second part. QUESTION 3. I just need a way to synthesize the lactone A, I already started please continue from where I left it Second image: I simply need the products, substrates or reagents, thank youarrow_forwardIndicate how to prepare a 10% sodium hydroxide (NaOH) solution to a slightly alkaline pH.arrow_forward
- CH, CH CH₂ CH₂ Phytyl side chain 5. What is the expected order of elution of compounds A-D below from a chromatography column packed with silica gel, eluting with hexane/ethyl acetate? C D OHarrow_forwardPlease analze my gel electrophoresis column of the VRK1 kinase (MW: 39.71 kDa). Attached is the following image for the order of column wells and my gel.arrow_forward2.0arrow_forward
- Write the electron configuration of an atom of the element highlighted in this outline of the Periodic Table: 1 23 4 5 6 7 He Ne Ar Kr Xe Rn Hint: you do not need to know the name or symbol of the highlighted element! ☐arrow_forwardCompare these chromatograms of three anti-psychotic drugs done by HPLC and SFC. Why is there the difference in separation time for SFC versus HPLC? Hint, use the Van Deemter plot as a guide in answering this question. Why, fundamentally, would you expect a faster separation for SFC than HPLC, in general?arrow_forwardA certain inorganic cation has an electrophoretic mobility of 5.27 x 10-4 cm2s-1V-1. The same ion has a diffusion coefficient of 9.5 x 10-6cm2s-1. If this ion is separated from cations by CZE with a 75cm capillary, what is the expected plate count, N, at an applied voltage of 15.0kV? Under these separation conditions, the electroosmotic flow rate was 0.85mm s-1 toward the cathode. If the detector was 50.0cm from the injection end of the capillary, how long would it take in minutes for the analyte cation to reach the detector after the field was applied?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





