
Concept explainers
Interpretation:
The four identified
Concept Introduction:
When the carboxyl functional group is bonded between two hydrogen atoms or one hydrogen atom and one alkyl group, then the compound is called an aldehyde. The general formula of aldehydes is drawn here:

When the carboxyl functional group is bonded between two alkyl groups, then the compound is called a ketone. The general formula of ketones is drawn here:
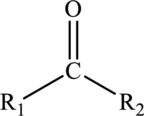
Carboxylic acids:
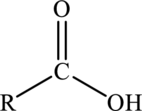
Carboxylic acids are hydrocarbon derivatives that contains the carboxyl functional group bonded to the alkyl groups. The general formula of carboxylic acids is drawn here:
Ethers:
Ethers are organic compounds in which the
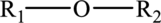
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
- List the functional groups in the structures below:arrow_forwardName 5 common organic compounds that are found in your home. Draw their Lewis structures and give their molecular formula. Encircle and identify the functional group/s that is/ are present in each molecule. Write your answers in another sheet of paper.arrow_forwardSolve both questions.arrow_forward
- Identify the functional group in the following molecule. Your choices are alkene, alkyne, aromatic, alcohol, thiol, ether, aldehyde, ketone, carboxylic acid, ester, or amine.arrow_forwardAcetaminophen, a popular painkiller, has the following structure: Name the recognizable functional groups in this molecule. Do you think there are other groups of atoms in this molecule that might qualify as functional groups?arrow_forwardhe the molecule below. Check the box next to each functional group the molecule contains. In the last column, e mber of each group next to the functional group names you selected. If the molecule does not contain a listed nal group, do not check the box and leave the last column blank. HO HO OH OH Select all functional groups present in the molecule O alcohol aldehyde alkene alkyne O amide amine arene O carboxylic acid ester ether ketone How many # 40 ICI 0 0 0 0 0 0 0 0 0arrow_forward
- I need help with these questions. Did I name those correctly?arrow_forwardPlease convert the following skeletal/line drawings to full Lewis dot structures (including lone pairs). Write the formula. Identify any major functional groups in each structure.arrow_forwardAm i missing a functional group?arrow_forward
- when looking at the name of a molecule the suffix tells you whether there are any double or helpful in answering questions on the subsequent pages. triple bonds in the molecule and the root tells you how many carbon atoms are present. Your instructor will guide you as you fill in the following table. The information in this table will be Note the suffix in each of the following functional groups: Alkanes have only ● • Alkynes have Alkenes have 1 Note the root associated with each of the following number of atoms: # of Carbons Example 2 bonds between carbon atoms. bonds between at least two of the carbon atoms. bonds between at least two of the carbon atoms. Root CH4 H Name this res charge! ~ 3 m ㅋ o bonds Can't be righ-arrow_forwardGive the name of each of the indicated functional groups in the molecule below. H Ope 2. 1. 4. Do OH 5. 3.arrow_forwardFor the molecule shown below, provide the names for all of the indicated functional groups: 3 1 2 1. Functional group 1: 2. Functional group 2: 3. Functional group 3:arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER  Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning




