
Concept explainers
(a)
Interpretation:
The skeletal structure of
Concept Introduction:
Fatty acids are long-chain
Unsaturated fatty acids can be defined as the long-chain fatty acids which have a long hydrocarbon chain with −COOH group. In unsaturated fatty acids, the carbon chain must have at least one double bond.
Answer to Problem 34P
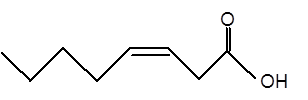
Explanation of Solution
Given:
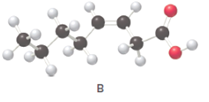
Fatty acids are long-chain carboxylic acids, which consist of two parts; long hydrocarbon chains and polar −COOH group. The long hydrocarbon chain consists of carbon and H atoms, so it has C-C and C-H bonds only, whereas, the polar −COOH group has polar C=O and C-O bonds. The unsaturated fatty acids have cis and trans-double bonds in the hydrocarbon chain.
In the ball-and-stick model, the red ball represents O atom, the black ball represents C atom and the white ball represents H atoms. Thus, the skeletal structure is:
(b)
Interpretation:
The omega-n designation for the
Concept Introduction:
Fatty acids are long-chain carboxylic acid, which may or may not have unsaturation in the molecule. They react with glycerol to form triglycerides. The reaction is called esterification as a carboxylic acid group reacts with the alcoholic group to form an ester group.
Unsaturated fatty acids can be defined as the long-chain fatty acids which have a long hydrocarbon chain with −COOH group. In unsaturated fatty acids, the carbon chain must have at least one double bond.
Answer to Problem 34P
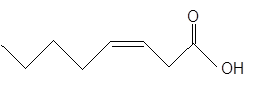
The
Explanation of Solution
Fatty acids are long-chain carboxylic acids, which consist of two parts; long hydrocarbon chains and polar −COOH group. If there is at least one double bond in the long hydrocarbon chain of the fatty acid, it is said to be an unsaturated fatty acid.
The unsaturated fatty acids can also classify as omega-n acids. Here, 'n' represents the position of the first
In the given fatty acid
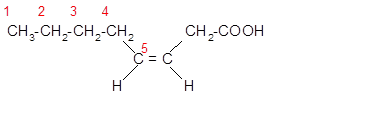
(c)
Interpretation:
The stereoisomer of the
Concept Introduction:
Fatty acids are long-chain carboxylic acid, which may or may not have unsaturation in the molecule. They react with glycerol to form triglycerides. The reaction is called esterification as a carboxylic acid group reacts with the alcoholic group to form an ester group.
Unsaturated fatty acids can be defined as the long-chain fatty acids which have a long hydrocarbon chain with −COOH group. In unsaturated fatty acids, the carbon chain must have at least one double bond.
Answer to Problem 34P
The stereoisomer of cis-fatty acid must be trans-isomer as given below;
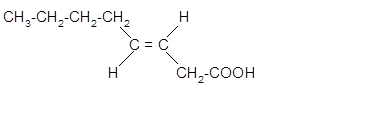
Explanation of Solution
Fatty acids are long-chain carboxylic acids, which consist of two parts; long hydrocarbon chains and polar −COOH group. The double bond can arrange in two ways, i.e., cis and Trans in the hydrocarbon chain of the fatty acid molecule. Hence, the stereoisomer of cis-fatty acid
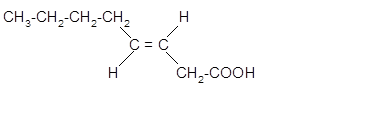
(d)
Interpretation:
The structure of wax formed by the reaction of
Concept Introduction:
Fatty acids are long-chain carboxylic acid, which may or may not have unsaturation in the molecule. They react with glycerol to form triglycerides. The reaction is called esterification as a carboxylic acid group reacts with the alcoholic group to form an ester group.
Unsaturated fatty acids can be defined as the long-chain fatty acids which have a long hydrocarbon chain with −COOH group. In unsaturated fatty acids, the carbon chain must have at least one double bond.
Answer to Problem 34P
The skeleton formula of wax formed by the reaction of
Explanation of Solution
Fatty acids are long-chain carboxylic acids, which consist of two parts; long hydrocarbon chains and polar −COOH group. Waxes are a good example of hydrolyzable lipids, which are composed of fatty acid and higher alcohols. They have an ester
Hence, the reaction of
Want to see more full solutions like this?
Chapter 19 Solutions
GENERAL,ORGANIC, & BIOLOGICAL CHEM-ACCES
- Calculate the equilibrium constant at 25.0 oC for the following equation. Cd(s) + Sn+2(aq) ↔Cd+2(aq) + Sn(s) Group of answer choices 3.11x104 1.95x1018 9.66x108 1.40x109arrow_forwardWhat is the pH at the cathode for the following cell written in line notation at 25.0 oC with a Ecell = -0.2749 V? Ni(s)|Ni+2(aq, 1.00 M)||H+1(aq, ?M)|H2(g, 1.00 atm)|Pt(s)arrow_forwardCalculate Ecell for a hydrogen fuel cell at 95.0 oC using the following half-reactions with PH2 = 25.0 atm and PO2 = 25.0 atm. O2(g) + 4H+1(aq) + 4e-1 → 2H2O(l) Eo = 1.229 V 2H2(g) → 4H+1(aq) + 4e-1 Eo = 0.00 Varrow_forward
- Calculate Ecell at 25.0 oC using the following half-reactions with [Ag+1] = 0.0100 M and [Sn+2] = 0.0200 M. Ag+1(aq) + 1e-1 Ag(s) Sn+2(aq) + 2e-1 Sn(s)arrow_forwardDone 18:19 www-awu.aleks.com Chapter 12 HW Question 27 of 39 (5 points) | Question Attempt: 1 of Unlimited .. LTE סוי 9 ✓ 20 ✓ 21 × 22 23 24 25 26 27 28 29 30 Answer the following questions about the given alkane. Part: 0 / 2 Part 1 of 2 Classify each carbon atom as a 1º, 2º, 3º, or 4°. Highlight in red any 1° carbons, highlight in blue any 2° carbons. highlight in green any 3° carbons, and leave any 4° carbons unhighlighted. Skip Part Check Save For Later © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center | Accessibility ☑ คarrow_forward< Done 19:22 www-awu.aleks.com Chapter 12 HW Question 4 of 39 (2 points) | Question Attempt: 5 of Unlimited : .. LTE סוי 1 ✓ 2 ✓ 3 = 4 ✓ 5 ✓ 6 ✓ 7 ✓ 8 ✓ 9 = 10 11 ✓ 12 Consider the molecule (CH3)2CHCH2CHCн for the following questions. Part 1 of 2 Which of the following molecules is/are constitutional isomer(s) to (CH3)2CHCH2CH2CH3? Check all that apply. Part 2 of 2 (CH3),C(CH2)2CH3 CH3 H,C-CH-CH-CH, CH 3 None of the above. ☑ Which of the following molecules is/are identical molecules to (CH3)2CHCH2CH2CH₁₂? Check all that apply. CH3 H,C-CH-CH₂-CH2-CH, CH3(CH2)2CH(CH3)2 CH2-CH2-CH3 HỌC-CH=CH, 乂 ☑ а None of the above Check Save For Later Submit Assignment © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forward
- 18:11 LTE ا... US$50 off hotels is waiting for you Book now, hotels in Nashville are going fast QUTSLIVII 25 61 69 points) | QuestIVIT ALLēm... now Give the IUPAC name for each compound. Part 1 of 3 Part 2 of 3 X ☑ Х Check Save For Later Submit © 2025 McGraw Hill LLC. All Rights Reserved. TOMS CT US ...vacy Center | Accessibilityarrow_forwardDone 19:17 www-awu.aleks.com Chapter 12 HW Question 29 of 39 (6 points) | Question Attempt: 1 of Unlimited .III LTE סוי 27 28 = 29 30 31 32 = 33 34 35 Consider this structure. CH3CH2CH2 Part 1 of 3 3 CH2 CH2CH3 - C-CH2CH 3 H CH₂ Give the IUPAC name of this structure. 3-ethyl-3,4-dimethylheptane Part: 1/3 Part 2 of 3 Draw the skeletal structure. Skip Part < Check Click and drag to start drawing a structure. Save For Later Submit © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Хarrow_forward18:57 .III LTE www-awu.aleks.com Chapter 12 HW Question 31 of 39 (8 points) | Question Attem... Give the IUPAC name of each compound. Part 1 of 4 Part 2 of 4 Х Х Check Save For Later Submit © 2025 McGraw Hill LLC. All Rights Reserved. TOMS OF US vacy Center | Accessibilityarrow_forward
- What is the missing reactant in this organic reaction? CH3-C-CH2-NH2 + R - CH3 O: 0 CH3-N-CH2-C-NH-CH2-C-CH3 + H2O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. Explanation Check Click anywhere to draw the first atom of your structure. C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forwardDone 18:17 • www-awu.aleks.com Chapter 12 HW Question 24 of 39 (4 points) | Question Attempt: 1 of Unlimited ▼ 20 ✓ 21 × 22 23 24 25 26 raw the structure corresponding to each IUPAC name. Part 1 of 2 .III LTE 22 27 28 סוי 29 29 3 A skeletal structure corresponding to the IUPAC name 3-ethyl-4-methylhexane. Part 2 of 2 Click and drag to start drawing a structure. A condensed structure corresponding to the IUPAC name 2,2,4- trimethylpentane. Click anywhere to draw the first atom of your structure. Check Save For Later Submit < Х ப: G © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility : Garrow_forwardDone 18:25 www-awu.aleks.com .III LTE Chapter 12 HW Question 29 of 39 (6 points) | Question Attempt: 1 of Unlimi... Oli 23 24 25 26 27 28 29 30 Consider this structure. CH2 CH2CH2 CH2CH2CH₂ C -C. -CH2CH3 H CH Part: 0 / 3 Part 1 of 3 Give the IUPAC name of this structure. Skip Part < Check ☑ Save For Later © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility ....................arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





